

Library Lobby | Main Index | Search | Publication List
AGRO-BIO - 370 - 04E
I would like to thank Christiane Trudeau of the McGill University Institute of Parasitology, Jean-Marie Boucher of the Centre d'Agriculture Biologique de la Pocatière and consulting agronomist Caroline Morin for their comments on the text.
Internal parasites in ruminants constitute a problem that returns periodically in almost all livestock herds. Recourse to synthetic dewormers is only a short-term solution. Animals that graze are always exposed to parasites and are thus constantly being reinfected; not to mention that routine deworming treatments delay the development of immunity in young animals. Moreover, certain parasites have developed a resistance to such deworming products as Benzimidazole, Levamisole and even Ivermectin because of too frequent use. Studies in New Zealand and Ireland indicate among other things that dewormers slow the decomposition of manure.
A serious pest control program in organic farming begins with a good understanding of parasites and the implementation of preventive measures. The ultimate objective is to develop an animal production system where parasites may be present in small numbers but do not affect the health or performance of herds. Deworming treatments, whether administered using natural products or not, should therefore only be employed in emergency situations or, if using weaker products, as preventive maintenance.
This document provides a description of internal parasites, methods to prevent their infestation and alternatives to conventional dewormers for ruminants. The section on botanical dewormers also includes information on other farm animals (pigs and poultry).
Knowledge of the life cycle and characteristics of parasitic worms is essential for anyone wishing to reduce their use of dewormers. Figure 1 shows the direct life cycle common to most parasites. Some parasites however have an indirect cycle, which involves a host animal. For example, the liver fluke (Fasciola sp.) spends part of its life in certain snail species before infecting ruminants. Table 1 presents the main classes of internal parasites, whereas Table 2 provides a list of the main internal parasites of ruminants and their characteristics. Internal parasites are most often worms (helminths) but can also be protozoa, which will not be covered here. Worms are generally specific to one organ, such as the abomasum, duodenum, and lungs.
1. Parasite-infested animal harbours adult worms...
2. The eggs produced by the female are deposited in pastures with fecal matter...
3. They develop into various larvae stages.
4. Animal is contaminated by absorbing L3 larvae or infesting larvae with the grass.
5. The larvae make their way to the alimentary canal where they develop and produce a new generation of adult male or female parasites.
Figure 1. Life cycle of internal parasites in ruminants
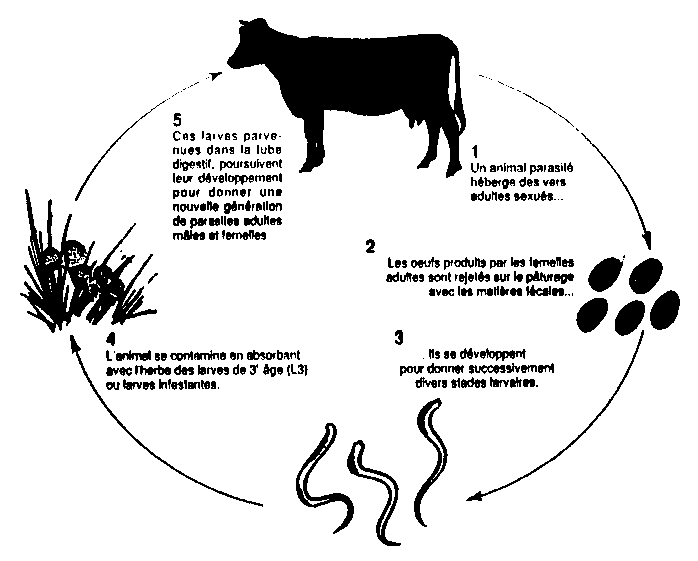
Source: Rivard et Huneault (1992)
Table 1 - Major classes of internal parasites
| ROUNDWORMS or Nemathelminthes | |
| Strongyles | |
| Gastrointestinal worms | |
| Abomasum worms: Haemonchus, Trichostrongylus, Ostertagia | |
| Duodenum worms: Trichostrongylus, Nematodirus, Cooperia, Strongyloides | |
| Large intestine worms: Oesophagostomum, Trichuris | |
| Small intestine worms or Ancylostomidae (hookworm): Ancylostoma, Necator, Bunostomum | |
| Lungworm or metastrongyles: Dictyocaulus, Metastrongylus, Protostrongylus | |
| Ascarids: Ascaris (duodenum) | |
| FLATWORMS or platyhelminthes | |
| Cestodes (tapeworm): Taenia, Echinococcus, Moniezia (duodenum) | |
| Trematodes: Fasciola, Dicrocoelium (liver) | |
Table 2 - Characteristics of main internal parasite genera in cattle, sheep and goats
| Parasite | Description | Infected Organ | Life Cycle | Symptoms |
| Haemonchus | M: 10-20 mm red F: 18-30 mm red and white | Abomasum | IS: 4-6 days PP: 3 weeks | Anemia, soft swelling under jaw and abdomen, weakness, no weight gain |
| Ostertagia | M: 6-9 mm, brown F: 8-12 mm | Abomasum | IS: 4-6 days PP: 3 weeks | Same as Haemonchus and also lack of appetite, diarrhea |
| Trichostrongylus | M: 4-5.5 mm F: 5-7 mm light brown | Abomasum, duodenum | IS: 3-4 days PP: 2-3 weeks | Same as Haemonchus and also diarrhea and weight loss |
| Cooperia | red M: 5-7 mm F: 6-9 mm | Duodenum | IS: 5-6 days PP: 15-20 days | Same as Haemonchus |
| Bunostomum | 10-30 mm | Duodenum | IS: ? PP: 30-56 days | Edema, anemia, weight loss, diarrhea |
| Strongyloides (young animals) | 4-6 mm | Small intestine | IS: 1-2 days PP: 8-14 days | Anorexia, enteritis, diarrhea |
| Chabertia | M: 13-14 mm F: 17-20 mm | Large intestine | IS: 5-6 days PP: 42 days | Anemia, diarrhea with blood |
| Oesophagostomum | M: 12-17 mm F: 15-22 mm | Large intestine | IS: 6-7 days PP: 41-45 days | Dark green diarrhea edema |
| Protostrongylus | M: 16-28 mm F: 25-35 mm | Lungs | IS: 12-14 days PP: 30-37 days | Pneumonia |
| Dictyocaulus | M: 30-80 mm F: 50-100 mm | Lungs | IS: 6-7 days PP: 3-4 weeks | Sticky nasal discharge, difficulty breathing, cough |
Legend: M = Males; F = Females; IS = Infectious stage: minimum number of days for parasite to reach infectious larvae stage (L3) after hatching of eggs; PP = Prepatent stage: period up to appearance of first eggs in dung after host is infected.
One may ask what the role of internal parasites is in nature. Do they reduce populations that are too large for the resources available? Overpopulation is often, if not always, inherent to agriculture. Or do parasites serve to cull the weaker animals, thus reinforcing a species' chances for survival? This is doubtful since it is generally not to the parasite's advantage to kill its host.
Whatever the case, it is normal in nature to find internal parasites in animals. In a natural setting, ruminants, although in herds, are constantly moving from one grazing area to another. It is therefore rare that the soil and grasses they eat are highly contaminated. Since levels of infestation are rarely excessive, animals have a chance to develop immunity.
The levels of infestation in goats and sheep that are raised in fairly natural settings tend to fluctuate with seasonal metabolisms without the animals being treated25. It has been observed in goats and sheep that the highest levels of parasites correspond to periods of change: change in location (e.g. buildings to pasture in the spring); change in diet or use of food (e.g. lactation to maintenance diet). This would indicate that internal parasites may play a role in helping animals get through periods of change and adaptation.
The point of view of biodynamic practitioners is focused largely on the role parasites play in animal digestion36. They believe that gastrointestinal parasites play a role similar to earthworms in the soil; that they "aerate" the digestive system when it is overloaded by too much silage, grain or green hay. Since the roots of plants have the same effect as earthworms, biodynamic farmers add fodder roots (e.g. beets, carrots) to animal feed to replicate the role of parasites.
An animal without worms is not an ideal to strive for at any cost, at least not in organic farming. An animal that never has worms can not develop resistance and is thus extremely vulnerable when exposed to a parasite. Resistance or immunity is the ability to prevent or limit the establishment or subsequent development of worm infections. Tolerance is the ability to maintain good productivity despite infection. Contrarily, susceptibility to parasites is defined by how easily the animal becomes infected.
Ideally, grazing animals - especially the youngest ones - should ingest parasites in small quantities so that they may progressively develop immunity. This does not apply, however, to all internal parasite species.
Susceptibility according to species
Most internal parasites are specific to one or two species. When they are found in other animals, it is usually only for a brief period. Certain parasite species common to several types of domestic animals have even developed more specific "breeds".
Although there are just as many parasite species that can infect cattle, sheep or goats, sheep are the most susceptible to internal parasites because they graze close to the ground. Goats and sheep, whose manure is in pellet form, graze directly over their manure, which makes them more susceptible than cattle who do not have access to the grass under their pats. Also, cattle tend to avoid the less appetizing grass near the pats.
Susceptibility according to age
The age as well as the weight of animals determine susceptibility to parasites. Young animals do not have a great deal of immunity to parasites during their first year at pasture. The second year, they have partial immunity and, although they may appear healthy, they excrete many eggs. Adult animals are much less susceptible to most parasites, unless they are in poor living conditions. For example, it has been demonstrated that horses 15 years of age or older are rarely infected by strongyles13. Instead, parasites like Strongyloides are almost exclusively found in young animals.
Other susceptibility
Animals are sometimes kept in conditions that make them highly susceptible to parasites. In the case of a recently dewormed animal, internal parasites no longer exist. There is thus no equilibrium and such an animal put into a contaminated pasture may be seriously affected. Animals in poor condition (e.g.: recent illness, food shortages) are also highly susceptible.
Genetic resistance
There are breeds or lines of animals that are resistant or more tolerant to internal parasites. In New Zealand, herds of sheep resistant to internal parasites were developed from Romney sheep. The approach adopted by organic farmers in New Zealand is to over the years develop a herd that is increasingly resistant, using resistant rams only, and not ewes.
The first step in a pest control program is to assess the situation. The two methods used for this purpose are fecal counts and field counts.
Veterinary offices conduct fecal analyses. These consist in identifying the species of parasites present in the animal and counting the eggs of the parasites per gram of stool. Results of the analyses are often expressed in qualitative terms: absence of parasites, low, average or high levels. In all cases, it is important to identify the parasite. Two approaches may be used:
Herd analysis
Randomly selected feces are used to determine the general state of the herd. A minimum of three to five pats is required in the case of cattle. Ideally the stools should be collected at midday for the egg production is more uniform at this time8.
Individual analysis
Feces from a single animal are used by isolating the animal and collecting them first thing in the morning and then fresh stools during the day. The purpose of an individual analysis is to confirm that the symptoms observed in the animal are in fact caused by a parasite infection.
Stool analyses have limits as methods for evaluating the situation. Certain species of parasites lay few eggs, others many. Some lay eggs only at certain times of the year or during a particular period in the ruminant's life cycle. The best way to benefit from fecal counts is to always perform them at the same time each year and preferably during critical periods, such as when the animals are put out to pasture or before bringing them in for winter. If the parasite level is high, two to four analyses will provide a better picture of the situation. Comparison from one year to another of analyses conducted during the same period will indicate if there is an improvement or not.
Other instances where stool analyses are useful are, for example, when there is a change of pasture ground, when new animals arrive, or when there are animals who appear to be ailing or young animals that are not putting on weight.
A field count is more difficult to do. In North America, it is mostly done as part of research programs. Samples that are representative of the grazed pasture must be collected, taking into account the height of the cut. In New Zealand, where this type of analysis is more common, it is considered with respect to sheep that if there are less than 100 larvae per 100 kg of grass there are neither economic losses nor drops in productivity39.
From the ecological perspective, serious problems with internal parasites indicate that changes in feed, field management or soil management are required. By changing the production method or by using preventive measures, it is not necessary to rely on dewormers too often.
An animal is better able to resist or tolerate internal parasites when its living conditions are good. Links between diet, particularly vitamins and minerals, and susceptibility to internal parasites have been established in certain cases. Vitamins A, D and B complex are the most important vitamins required by animals to develop resistance to internal parasites23. Nyberg and his coworkers, quoted by Quiquandon31, have established that a lack of cobalt promotes parasitism, since cobalt is the element used by animals to synthesize vitamin B12. Iron supplements are also very important where animals are affected by worms that drain the blood, like Haemonchus (worms in the abomasum) and Ancylostoma (intestinal worms). According to Lapage23, animals should always have access to mineral blocks to compensate for the mineral deficiencies in pastures. In barns, animals should be fed from feeders rather than directly from the ground to avoid contamination as a result of their mouths coming into contact with manure or bedding.
At the New Zealand Pastoral Agriculture Research Institute, research is currently underway on how different foods affect resistance to parasites. Forage crops that contain condensed tannins, like trefoil, allow animals to better fight parasites than others that do not contain any, like alfalfa.
The age at which young animals are weaned is an important factor in regard to parasite resistance. For example, it has been observed that milk-fed calves are distinctly less contaminated by Haemonchus, Cooperia and Oesophagostomum than weaned calves34. Milk does not have any effect, however, on Ostertagia and Trichostrongylus infections.
Ideally, females should calve during periods when risk of contamination is low, so that young animals are exposed as late as possible to potentially contaminated pastures. In Northern parts of North America, the winter period seems most appropriate in this respect. All new arrivals to the herd should be quarantined for four to six weeks and dewormed if there is any doubt.
Pasture management that is designed to prevent internal parasites requires long-term planning. It is by varying such factors as the density and age groups of animals and the time and intensity of grazing that serious infections can be avoided.
Animal density
Overpopulation increases the concentrations of parasites. It is generally estimated that parasite infections increase with the square of the animal load per surface unit. Therefore, for a given parcel of land, parasite infestations are four times greater where animal density is doubled. Density varies depending on whether grazing is intensive or extensive. Where there is extensive grazing, Antoine2 recommends less than 10 lambs/ha (varying according to context).
Pasture rotation, or intensive grazing, consists in dividing the pastures into parcels of land of varying sizes called paddocks and frequently moving the animals from one paddock to another to optimize grass use. From a parasitic point of view, the objective is to not put the animals back into the same field until the risk of infection has diminished. Theoretically this means that parasitism will decrease if the number of parcels of land is increased or the rotation time is increased. Unfortunately, in practice, it appears difficult to diminish the parasitic load with intensive grazing. The lifespan of L3 larvae is in fact always greater than the time required between grazing periods for maximum grass use. Therefore, if one waits six weeks before returning animals to a lot, the quality of the grass decreases as well as the quantity of grass ingested by the animals, whereas the level of parasites only diminishes slightly.
Grazing height
About 80% of parasites live in the first five centimetres of vegetation. Parasite infection and multiplication are prevented by letting animals graze only 10 cm from the ground in a field where there are parasites. For new pastures, however, New Zealander Vaughan Jones18, an expert on intensive grazing, recommends having animals graze very close to the ground, so that the sun can dry the pats quickly and thus diminish the chances of survival of parasites brought in with the animals. A new pasture is considered a field where animals have not been grazing for a number of years. It may be a pasture seeded in the spring or a hay or silage field that is used as pasture after harvest.
Grazing time
The drier the grass, the more parasites will stay at the base of the plants. It is estimated that in wet grass, larvae can be found over 30 cm away from the pats, whereas they venture only a few centimetres away when the grass is dry. The risk of infection is greatly lowered by waiting until the dew has lifted or until the grass has dried after rain before putting animals out to pasture.
The larvae of most parasites move to the tops of plants when light levels are low, that is, when the sky is overcast or at sunrise and sunset. They avoid strong light however. Limiting grazing time to when the sun is strong also diminishes the risk of infection.
Since we know that the density of L3 larvae is generally at a maximum in the fall and at a minimum in the summer, it is preferable to limit grazing in highly contaminated fields to the summer months to reduce levels of ingestion. In the fall, the animals should ideally be put in a new pasture.
Risks of infection from most parasites can be avoided in the spring by waiting until the end of spring to put cattle out to grass, and even later in areas where soil drainage is poor. In practice, this solution is not very satisfactory, both economically and ecologically, because it implies shortening the grazing season, which is quite short as it is in most northern regions, and feeding the animals more hay.
Harrowing Pastures
With respect to parasites, harrowing pastures is generally not recommended. The parasite eggs and larvae are in fact scattered throughout the pasture. This makes it impossible for the animals to graze selectively, that is, to graze around the pats. Harrowing would be appropriate, however, at the beginning of a dry period in a field that the animals will not be returning to for quite some time.
Biodynamic practitioners have a different point of view. They consider that parasites proliferate in an environment that is nitrogen-rich and sheltered from light. They therefore recommend breaking up the pats to let in air and light.
Grazing by age group
Since the susceptibility of animals varies with age, it is logical to graze the younger animals in fields where parasite populations are very low.
Organic farmers in New Zealand have established some rules to prevent internal parasites in lambs and ewes. Thus, in intensive grazing, lambs do not have access to paddocks or sections of a pasture already grazed by ewes or other lambs in order to prevent reinfection. Lambs should graze preferably in new pastures, hay or silage fields, or should be greenfed.
Since sheep graze year-round in New Zealand, there is usually an increase in parasites in the spring, due to a drop in immunity in ewes after lambing. Parasite levels rise again at the end of the summer to early fall. Consequently, ewes do not go out to pasture until the lambs are weaned. After weaning, the ewes graze in a different part of the farm while alternate groups of lambs graze in another sector. These sectors are rotated each year.
Another New Zealand technique is used to reduce parasite infection in calves. This technique consists in grazing the calves alone or in pairs on a rotating basis. The calves remain in the same lots all the time while the cows are rotated. Very good results have been obtained this way, even though there is no clear reason why this method works.
Another common practice used with calves is to put them in a new pasture. To fight Ostertagia infection, for instance, the calves would be placed in an old field at the beginning of the season, then dewormed and brought to a parasite-free field in early July.
In dairy herds, young cows can be slowly immunized by allowing them to graze in new pastures with two dry cows that serve as sources of infection. The ingestion rate of L3 larvae is therefore quite low, allowing for controlled infection and development of immunity.
Multispecies grazing
Producers who have more than one animal species (e.g. cattle and sheep) can alternate grazing of different animal species which, although not foolproof, can help to break the parasite cycles. Several parasite species cannot infect two different animal species. There are even certain species of worms that affect only a particular ruminant species.
Cattle and sheep herds can be combined in three ways:
(1) Graze the cattle to "clean" the pasture after the lambs have grazed. The cattle ingest a significant quantity of mature larvae from the lamb stools. If the cattle are allowed to graze the grass down to 3 to 5 cm from the ground, many parasites will be killed off from exposure to the sun;
(2) Graze the cattle before the sheep to control pasture quality;
(3) Graze the cattle and sheep together where vegetation is abundant.
Deworming treatments have little effect if the animals are returned to the same larvae infested field. It is therefore important to clean the pasture as much as possible to reduce, if not eliminate, the parasites. Possible strategies for this are resting the land, planting, using amendments or fertilizers to reduce parasite populations, and improving drainage.
Resting the land
This consists in preventing the animals from grazing in the same field or paddock. Since freezing temperatures or droughts eliminate some infectious larvae, cold or dry periods can be relied upon to reduce or extend rest periods. A three-year rest period (short rotation) is required for a complete cleaning.
Nematicide plants
Mustard is an excellent nematicide plant and so are tagetes. For more information on the subject, consult the Agro-Bio synthesis entitled "Controlling nematodes with nematicide plants".
Amendments and fertilizers
Amendments that change the pH, the mineral balance or that create an environment which is inappropriate for parasites may help to clean the land. The choice of amendment or fertilizer depends on the type of parasite. According to Nunnery26:
Manure management
Manure to be used for spreading may be filled with parasite eggs and larvae. Composting is a good way to clean manure as the larvae and eggs of nematodes are destroyed at temperatures as low as 32 to 34C. They are killed in as little as one hour at 50C, and in less than four hours at 44C. It is important, when turning the compost over, to ensure that the outer layer which has heated less, be mixed towards the middle of the pile. Composting is a useful technique before manure spreading in the case of truly dangerous parasites like lungworm.
Bedding is also important. For example, it has been demonstrated that eggs and larvae are all killed in horse manure mixed with straw bedding, as well as horse manure mixed in a one to four ratio with cow manure3.
Parnell28,29 studied the effects of adding different nitrogen fertilizers to, among others, sheep and horse manure. Urea was the most efficient nitrogen fertilizer for cleaning manure, with a required proportion of 1:125. Kainite (sulphate of potassium and sodium) was the most efficient non-nitrogen fertilizer to add to manure, with a required proportion of 1:23. In practical terms, Parnell suggested applying these substances to the surface only, since temperatures in the middle of the manure pile would be high enough to eliminate the parasites.
Improving drainage
Pastures or parts of pastures that remain wet for long periods are an ideal environment for the survival of internal parasite larvae. Standard drainage of a field may reduce the larvae's chances of survival and extend grazing periods. Localized drainage of wet patches prevents infectious larvae from persisting in an otherwise clean field. It is also important that cattle watering areas be situated in well-drained places with gravel or even cement added. Animals must be prevented from accessing swamps or streams at all costs because of the parasitic risks, the damage the animals cause to these areas and the risks of pollution.
When to deworm
It is crucial to choose the right time to carry out deworming treatments. At a certain stage of their development inside the animal, some parasites embed themselves in the mucous membranes and enter hypobiosis (e.g. Ostertagia). They are largely inactive at this stage and relatively harmless to the host. Deworming treatments have little or no effect when performed at this time.
A sensible conventional practice to employ against parasites is to perform a first treatment three weeks after the animals have been put out to pasture and a second treatment three weeks later. The first treatment serves to prevent infection by infectious larvae (L3 stage) - before the new adults formed inside the animals have begun to lay eggs profusely and contaminate the pastures. When the second treatment is given, typically in early July, a large portion of the infectious larvae in the pastures will have died as a result of the hot dry conditions.
According to a traditional French practice, deworming treatments are performed preferably when there is a new moon. The worms are more active at this time and therefore easier to dislodge. On the other hand, Rudolf Steiner, the father of biodynamic agriculture, recommends performing deworming treatments during a full moon.
Lapage23 suggests deworming at the approach of dry or cold periods to benefit from the sterilizing effect of these factors.
Which animal to deworm
Antoine2 recommends deworming:
How to deworm
All deworming treatments involving natural products should ideally be preceded and followed by a fasting period, except in the case of nursed young animals. Animals should not be fed for a period of 12 to 48 hours before the treatment and another 6-hour period afterwards. A laxative diet or purge should then follow. Castor oil is appropriate for non-ruminants, and a saline diuretic or sodium sulphate and magnesium for ruminants. Liquid deworming treatments that animals do not willingly ingest can be administered using a funnel and a flexible tube put down the animal's throat, or a "gun" designed for this purpose.
In the case of milking dairy cows, it is difficult to fast the animals. Subsequently, it may be simpler to lighten their diet by not using silage or concentrates rather than to fast them. Note that putting animals out to pasture in the spring has a laxative effect on them. It seems appropriate therefore to treat the animals at that time.
For confined animals, a radically different way to deworm is to spray essential oils using an atomizer in order to fill the air with aerosols that have anthelminthic (synonym with dewormer) properties. Gape-infested pheasants have successfully been treated with pyrethrum oil, goosefoot oil and roto-resin using that method15.
Several plants have anthelminthic properties, and were in fact a part of the traditional husbandry before synthetic dewormers were commonly adopted. In Québec, for instance, it was common practice to feed evergreen branches (pine, spruce or fir branches) to sheep. Although based on conventional wisdom, veterinary research zeroed in on deworming plants, also called anthelminthic plants, particularly before the Second World War in Western countries then, subsequently, mainly in Eastern countries and India. There is reliable data available on the effects of several plants or plant extracts on certain parasites, enabling us to know the limits of these substances.
Allopathy versus homeopathy
Several of the dewormer plants mentioned below may cause side effects in animals. The most powerful natural dewormers are often potential poisons. It is therefore important to follow the indicated dosages. A way to avoid side effects is to administer these plants in the form of homeopathic preparations. The advantage of homeopathic remedies is that they do not require a fasting period beforehand and laxative diet after the treatment.
Garlic
Garlic is a common plant dewormer that is easy to find. It is known to be active against, among others, Ascaris, Enterobius and, of particular interest for ruminants, against lungworm in general1. It must be used, however, as prevention (prophylaxis) rather than as treatment or with other products. In fact, garlic does not prevent the production of eggs but prevents the eggs of certain parasites from developing into larvae5. In the ninth century, in Persia, Avicenne recommended the use of garlic as an additive rather than as a dewormer alone. Garlic is incorporated into certain commercial homeopathic or allopathic dewormers, but always with other plant-derived substances. The numerous therapeutic properties of garlic come mainly from its high sulphur content.
Garlic can be administered in several ways:
In the case of dairy animals, it is preferable to feed them garlic during or immediately after milking so that the milk does not pick up the taste.
Wormwood
Wormwood, as its name suggests, is an excellent dewormer. Many wormwood species have deworming properties.
- Common mugwort (Artemisia vulgaris) is effective against Protostrongylus, Dictyocaulus and Bunostomum. Sheep, goats and fowl readily consume it10.
- Common wormwood (Artemisia absinthium) must be used with caution as it may be dangerous if used regularly or excessively. The dried and crushed flowers may be used or steeped in cold water. De Baïracli-Levy9 suggests the following recipe for dewormer balls: four teaspoons of cayenne pepper powder, two teaspoons of powdered common wormwood mixed with honey and flour.
- Eurasian wormwood (Artemisia cina) is a desert plant that is used to make santonin and the homeopathic remedy Cina, which are used as dewormers. Santonin is extracted from the dried buds of the plant. The buds are then treated with liquid lime and dried again. Santonin acts against most parasites except Echinococcus. It must be used with caution, however, because even in small doses it causes side effects, particularly eye problems. Homeopathic Cina may be acquired as mother tincture, administered in 2 to 3 drops/10 kg, morning and evening for 3 weeks, or in granules in different dilutions. Consult a homeopathic veterinarian for more information.
- The dried, powdered shoots of Artemisia herba-alba wormwood (a species common to North Africa) administered in dosages of 10 to 30 g per goat proved highly effective against Haemonchus contortus17.
- Tarragon (Artemisia dracunculus) also has deworming properties. Several wormwood species grow wild in North America. It might be a good idea to let these plants grow along pastures where the animals can eat them as needed.
Wild ginger
Wild ginger or snakeroot (Asarum canadense) grows in wooded areas. This plant is very similar to European wild ginger which was used as an anthelminthic purge for cattle and horses. The dosage per animal is 20 to 30 g of the aerial parts of snakeroot mixed with wet bran. Wild ginger also has antibacterial properties. If planning to use this plant, remember that wild ginger, as well as wild garlic, require several years to reproduce.
Goosefoot
Chenopodium ambrosioides or goosefoot is a widely used dewormer plant. In Brazil, the plant is fed directly to pigs to deworm them. The powdered seeds serve as a dewormer and insecticide. The Japanese make a dewormer tea with the leaves. Oil from the goosefoot, although highly efficient, is extremely toxic. Human consumption has often led to strong side effects (nausea, headaches) and even death in some cases. It is better to use less hazardous substances than goosefoot oil.
Conifers
Conifers, like garlic, are undoubtedly more indicated in prophylactic form, that is, in small quantities in daily food, rather than as a curative treatment. In Russia, Ascaris infestations in pigs were reduced by giving them 1 to 2 kg of pine needles each day for 2 to 4 weeks39 and mixtures of conifer needle powder and sulphur or vitamins were also used successfully against internal parasites.
In practical terms, it is easier to use pitch, also called turpentine, extracted from pine and various other conifers. Turpentine spirits are a byproduct of turpentine distillation. Cabaret6 prescribes 50 to 100 ml of spirits produced from turpentine distillation with a triple volume of castor oil against ruminant liver fluke and horse strongyles. A mixture of linseed oil (edible and not the variety found in hardware stores) and turpentine spirits constitutes a powerful dewormer, but which must be used with caution. If turpentine enters the respiratory system it may cause the spasmodic closure of the mouth. It is therefore preferable to use rolled oats to soak up the turpentine before feeding it to animals. For one lamb, 10 to 15 drops of turpentine spirits are mixed with an ounce of linseed oil and a pinch of ground ginger; for an adult sheep, 80 drops in two ounces of linseed oil.
Common juniper (Juniperus communis) has deworming properties, notably against liver fluke. Sheep enjoy juniper berries and deer graze on the plant. It might be interesting to allow restricted access to woodlands where the animals can find conifers to eat if they wish.
Crucifers (mustard family)
White or black mustard seeds in the amount of 2 ounces per lamb is a safe dewormer, and it is recommended allowing the herd access to mustard in the pasture or elsewhere. In India, some cattle farmers use mustard oil against parasites in the amount of 100 to 150 g per day for one week. Mustard oil is more of a laxative than a dewormer, which is nevertheless useful in eliminating some parasites.
The following crucifers are dewormers and may be added to animal feed: radishes, raw grated turnips or horseradish, nasturtium seeds.
Cucurbits
The seeds of squash, pumpkins and many other vine crops contain a deworming compound called cucurbitacin that is more or less active depending on the parasite12. The seeds may be fed directly to animals as the Canadian pioneers once did, but it is better to extract the main ingredient using water, alcohol or ether, for an effect that is similar to that of pumpkin seeds. Aqueous extracts from squash seeds (dilution 1/50) are effective against Haemonchus contortus38.
Pumpkin seed dewormer24
Fern
The rhizomes and young shoots (fiddleheads) of the male fern (Dryopteris filix-mas) have deworming properties that have long been recognized in Europe, among others, against tapeworms (Taenia). The North American equivalent of the male fern is the evergreen shield-fern (Dryopteris marginalis). Although in the past, ether extract of the male fern was widely used against the liver fluke in the British Isles, the male fern does not give satisfactory results in the case of the Dicrocoelium fluke in sheep14, nor against Echinococcus in dogs.
The success of fern is enhanced by using fresh material and mixing it with glycerine. The male fern must be used with caution because it is toxic in high doses. In humans, for instance, it can cause headaches and nausea; the maximum dose is 7 g per adult.
Lupine
A diet made up entirely of freshly cut, lightly salted, lupine is a good dewormer that works against a large number of intestinal worms in pigs, including Trichuris (100% efficient), Strongyloides (66% efficient), Ascaris (50% efficient)7. Lupine is equally efficient against Parascaris and Strongylus in horses. It is important not to give free access to lupine, otherwise symptoms of poisoning may occur.
Nuts
Several vegetable species produce nuts that have anthelminthic properties, but unfortunately it is mostly tropical species like areca and cashew shells that are used. The fresh sap of the hazelnut (Corylus) is highly effective against Ascaris22.
Umbelliferae
Carrot seeds (Daucus carota), either wild or cultivated, are dewormers, as are teas made with the roots. A mixture of anise, cumin and juniper seeds is effective against Dictyocaulus lungworm in calves. Fennel leaves and seeds are also used as dewormers; the oil is a dewormer but very toxic too. In a central Asian area of the former USSR, it is common practice to graze sheep infected with Haemonchus in pastures where there are giant fennel (Ferula gigantea) and other Ferula species42. The worms are eliminated after two or three days and the plant is grazed for roughly 20 days. In many parts of North America, carrots and wild parsnips that grow abundantly along fields and roads could probably be used in the same way.
Pyrethrum
Pyrethrum (Chrysanthemum cinerariifolium) is commonly used as an insecticide in agriculture. It also has anthelminthic properties. As a dewormer, it is administered in powder form in animal feed. It may be safely used for warm-blooded animals, unless it is injected. In this case, it must be mixed with oil and the necessary precautions taken.
Pyrethrum is 100% effective against ascarids in chickens, in the amount of 200 mg/bird using 0.8% pyrethrum32. A complete cure was obtained against Ascaris in chickens, by giving them pyrethrum powder (concentration unknown), using 2% of the ration for 7 days44. Pyrethrum is also useful against strongyles in horses, in the amount of 3.5 mg/kg of live weight35. For more information on the veterinary uses of pyrethrum, see Urbain and Guillot41.
Although a Mediterranean plant, pyrethrum may be cultivated easily in many places. For more information on pyrethrum culture, see the Agro-Bio synthesis entitled "Home Production of Pyrethrum", available at EAP.
Tobacco
Tobacco and its derivatives (nicotine, nicotine sulphate) have been used as dewormers, particularly for fowl. With other farm animals, the mortal dose is practically the same for the worms as for the animals themselves!
Tansy
Tansy seeds (Tanacetum vulgare) are used against Nematodirus in sheep27. The oil from the flowers is also anthelminthic. An aqueous extract of tansy flowers and leaves is 100% effective in eliminating Ascaris from young horses and dogs, in the amount of 0.5ml/kg live weight in two doses administered one day apart and preceded by a one-day fast19. One kilo of leaves and flowers produces about one litre of extract. Cows and sheep consume fresh tansy easily, but goats, horses and pigs are not really fond of it.
Other plants
Blackberries, raspberries, and young ash and elder shoots are also other plant species with deworming properties that should be accessible in pastures. According to Cabaret6, beech creosote is used against lungworm in ruminants. The following plants, which grow naturally or may be cultivated in most of North America, are listed by Duke11 as having deworming properties:
A large number of tropical plants, and even some types of marine algae, also are reputed to have deworming properties.
Mixes
The commercial "natural" dewormer preparations, whether they are allopathic or homeopathic, are often mixes of different types of vegetation. The purpose of these mixes is to expand the range of action and, in some cases, they act, synergistically. Often, both tropical plants and those from temperate climates are included. Homeopathic laboratories each offer their own mixes, also called complexes. A list of major homeopathic laboratories is presented in the Useful Addresses section.
Diatomaceous earth
Diatomaceous earth is made from the remains of fossilized marine algae called diatoms. The product is mined and reduced to powder form. This powder acts as tiny pieces of glass that tear the shells of insects and other arthropods. Many farmers add diatomaceous earth to the rations of their animals, among others, because it contains minerals and is relatively inexpensive.
Some claim that diatomaceous earth acts as a dewormer when added on a regular basis in the amount of 2% of the ration. Scientific tests on the subject are limited however and opinions of farmers are contradictory. Moreover, diatomaceous earth has no effect on lungworm and is not very appetizing. It may also be a lung irritant. Given that the level of dust is already quite high in barns, diatomaceous earth does not seem appropriate when the animals are fed indoors. The main motivation for adding diatomaceous earth to rations should not be to control internal parasites. If it is to be used, it is important to use non-calcined diatomaceous earth and without additives for insecticide use. See the Useful Addresses section for the address of a supplier of diatomaceous earth for animal use.
Surfactants
Many American farmers use Shaklee's Basic H surfactant as cattle dewormer with success. However, the company does not endorse this use of the product. Also, the organic certification standards do not always allow its use because the exact nature of the product is a trade secret, although we know it is based on two soybeans enzymes. Grazier Joel Salatin from Virginia gives the product to his cattle through water at a rate of 1 cup of Basic H per 100 American gallons of water (a quarter cup into 100 litres) . He confines the animals for two days to make sure all the animals get it. Treatment is repeated 6 times a year, and costs less than 50 cents per head.
Copper sulphate
Copper sulphate, a mineral substance that already meets organic farming specifications for plant production, has a strong deworming action against certain parasites, particularly Haemonchus contortus and Trichostrongylus axei, which affect the abomasum. Copper sulphate is administered in a 1% solution in water, in the amount of 50 ml per lamb or 100 ml per adult sheep, and 30 ml/22.5 kg of live weight for calves to a maximum of 100 ml. This dewormer may be administered with a funnel and flexible tube. Treatment is given in the morning before the animals have eaten, followed by castor oil one half hour later. It is important not to feed animals for two hours following treatment because of poisoning risks.
Others
Peroxide and charcoal also have deworming properties according to some practitioners and salesmen. There is insufficient scientific data however to support these claims.
As to conclude, the following points can be highlighted from this review on alternative internal parasites control:
Alternatives to the utilization of synthetic dewormers exist to control internal parasites in ruminants. As is often the case in organic farming, we should aim not to eliminate these pests but to learn to coexist with them.
1. Anonymous. 1953. Garlic as an anthelmintic. Veterinary Record, 65(28):436.
2. Antoine, D. 1981. En élevage biologique faut-il déparasiter les animaux? Nature et Progrès, October/November/December 1981:12-16.
3. Antipin, D.N. 1941. [Research on deworming methods for horse and cow manures]. Vestnik Selsk. Nauki Veterinafiya, 1941 (2):42-56.
4. Baker, F.H. and R.K. Jones. 1985. Proceedings of a conference on multispecies grazing. Winrock International Institute for Agricultural Development, Morrilton, Arkansas.
5. Bastidas, G.J. 1969. Effect of ingested garlic on Necator americanus and Ancylostoma caninum. Am. J. Trop. Med. Hyg., 18(6):920-923.
6. Cabaret, J. 1986. 167 plantes Pour soigner les animaux. Editions du Point Vétérinaire, Maisons-Alfort, France. 192 pages.
7. Chebotarev, R. S. 1956. [The use of some fodder plants in the control of parasitic infections of farm animals]. Problemi Parazitologli, Transactions of the Scientific Conference of the Parasitologists of Ukrainian SSR, pages 194-197.
8. Cornils, W. 1935. Systematische Untersuchunger uber Strongylideneier und Strongyliden im Kot und Darminhalt des Pferdes. Berlin Dissert. 43 pages.
9. De Baïracli Levy, J. 1973. Herbal handbook for farm and stable. Faber and Faber, London.
10. Deschiens, R. 1944. Action comparée de la tanaisie et de l'armoise sur les formes larvaires de nématodes parasitaires et saprophytes. Bulletin de la société de Pathologie Exotique, 37(3/4) :111 -125.
11. Duke, J.A. 1985. CRC Handbook of medicinal herbs. CRC Press, Boca Raton, Florida, 677 pages.
12. Forgacs, P., J. Provost and R. Tiberghion. 1970. Etude expérimentale de l'activité anthelminthique de quelques cucurbitacines. Chim. Thér., 5(3):205-210.
13. Foster, A.O. 1937. A relationship in equines between age of host and number of strongylid parasites. Am. J. Hyg., 25:66-75.
14. Guilhon, J. 1956. Recherches sur le traitement spécifique de la dicrocoéliose ovine. Recueil de Médicine Vétérinaire, 132(10):733-749.
15. Guilhon, J. and J.P. Petit. 1960. Traitement de la syngamose des faisandeaux par les aérosols anthelminthiques. Compte-Rendu des Séances de l'Académie d'Agriculture de France, 46(7):1017-1020.
16. Grieve, M. 1971. A modern herbal. Volume 1. Dover, New York. 427 pages.
17. Idris, Um El A.A., S.E.I. Adam and G. Tartour. 1982. The anthelmintic efficacy of Artemisia herba-alba against Haemonchus contortus infection in goats. National Institute of Animal Health Quarterly, Japan, 22(3):138-143.
18. Jones, V. 1993. Grazing to control worms. The New Farm, 15(7):52-53.
19. Karamisheva, E.N. 1956. [Treating Parascariasis in foals and ascariasis in dogs with tansy]. Veterinariya, 33(12):29-30.
20. Kidd, R. 1993a. Roundup roundworms. The New Farm, 15(1):6-8.
21. Kidd, R. 1993b. Control parasites ornanically? The New Farm, 159(7):7-11.
22. Krotov, All. and D.G. Timoshin. 1957. [Trials of new preparations of vegetable origin against ascaridiasis in catsl. Meditsinskaya Parazitologiya i Parazitornie Bolezni, Moscow, 26(1):87-92.
23. Lapage, G. 1959. Mönnig's veterinary helminthology and entomology. 4th edition. Baillière, Tindall and Cox, London. 511 pages.
24. Lys. P., J Adès and Y. Badre. 1955. Essais sur les propriétés anthelminthique des graines de courge. Revue Médicale du Moyen-Orient, 12(3):339-340.
25. Mackenzie, D. 1967. Goat husbandry. Faber and Faber, London.
26. Nunnery. J. 1953. The control of internal parasites by the application of chemicals to the soil. Auburn Veterinarian, Alabama, 10(1):48-51.
27. Papchenkov, N.Y. 1968. [Tanacetum vulgare seed and naphtaman against Nematodirus infections in sheepl. Veterinarya Mosk., 45(8):48-49.
28. Parnell, l.W. 1937. Studies on the biodynamics and control of the bursate nematodes of horses and sheep. IV. On the lethal effects of some nitrogenous fertilizers on the free-living stages of sclerostomes. Canadian Journal of Research, Section D, 15(7):127-145.
29. Parnell, l.W. 1938. Studies on the biodynamics and control of the bursate nematodes of horses and sheep. V. Comparisons of the the lethal effects of some nonnitrogenous fertilizers on the free-living stages of sclerostomes. Canadian Journal of Research, Section D, 16(4)73-88.
30. Pena, N., A. Auro and H. Sumano. 1988. A comparative trial of garlic, its extract and ammonium Potassium tartrate as anthelmintics in carp. J. of Ethnopharm., 24(2-3): 199-203.
31. Quiquandon, H. 1978. 12 balles pour un veto. Tome II. 1ere partie. Éditions Agriculture et Vie. 239 pages.
32. Rebrassier, R.E. 1934. Pyrethrum as an anthelminthic for Ascaridia lineata. J. Amer. Vet. Med. Ass., 84:645-648.
33. Rivard, G and G. Huneault. 1992. Les parasites internes des bovins de boucherie. Bovins du Québec, August 1992:6-11.
34. Rohrbacher, Jr. G.H., D.A. Porter and H. Herlich. 1958. The effect of milk in the diet of calves and rabbits upon the development of trichostrongylid nematodes. Am. J. of Vet. Res., 19(72):625-631.
35. Rueda, E.A. 1954. [Comparison of pyrethrum and phenothiazine as anthelminthics anainst strongyles in horses]. Rev. milit. B. Aires, 2:147-152 and 154-156.
36. Salatin, J. 1994. Using Shaklee Basic H soap. Stockman Grass Farmer, 51(11):27-28.
37. Selinger, L. 1984. Cours d'élevage bio-dynamique. Mouvement de culture biodynamique, Huningue, France. 26 pages.
38. Sharma, L. D., H.S. Bahga and P.S. Srivastava. 1971. In vitro anthelmintic screening of indegenous medicinal plants against Haemonchus contortus of sheep and goats. Indian J. of Animal Research, 5(1):33-38.
39. Slepnev, N.K. 1967. [Use of some plants in the control of ascariasis in pigs]. Vetrinariya Moscow, 44(6):61-62.
40. Stieffel, W., J. Niezen and N. Thomson. 1992. Investigations into the control of intestinal parasites in lambs without the use of conventional anthelmintics. Pages 219 to 228 In Boehncke, E. and V. Molkenthin. 1992. Alternatives in animal husbandry. Proceedings of the International Conference on Alternatives in Animal Husbandry, Witzenhausen, 22-25 July 1991, Kassel University, Germany.
41. Urbain, A. and G. Guillot. 1931. Sur les pyréthrines et leur emploi en médecine vétérinaire. Rev. Path. Comp., 31 :493-502.
42. Utyanganov, A. A. and K.M. Yumaev. 1960. [The anthelmintic properties of Ferula plantsl. Veterinariya, 37(9):40-41.
43. Wall, R. and L. Strong. 1987. Environmental consequences of treating cattle with the antiparasitic drug ivermectin. Nature, 327:418-421.
44. Zarnowski, E. and J. Dorski. 1957. [Treatment of Ascaridia infestation in fowls]. Méd. Vét., Varsovie, 13:387-393.
© 1996 Ecological Agriculture Projects All rights reserved
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University
(Macdonald Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: info@eap.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster