

EAP Publications | Virtual Library | Magazine Rack | Search | What's new
Join the Ecological Solutions Roundtable
C. Arden-Clarke1 and R.D. Hodges 2
'The Political Ecology Research Group, 34 Cowley Road, Oxford OX4 1 HZ, U.K. Department of Biochemistry and Biological Sciences, Wye College
University of London , Wye, Ashford, Kent, TN25 5AH, U.K.
The inputs characteristic of conventional and organic/biological farming systems are examined, and their physical, chemical and biological impacts on the ecology and fertility of agricultural soils, and on nutrient cycles within these systems, are evaluated. Inorganic fertilisers applied in conventional systems may not preserve soil structure, can cause wide fluctuations in the pH and ion concentrations of the soil solution, and can substantially reduce some soil faunal populations, especially earthworms. Organic manuring practices characteristic of organic systems tend to maintain soil structure, are less disruptive of the soil chemical environment, encourage populations of beneficial soil fauna and contribute to the control of microbial pathogens.
Where inorganic fertilization practices fail to maintain soil organic matter levels, and therefore soil structure, they inhibit crop rooting and reduce the water retention capacity of the soil. Organic manuring practices generally facilitate crop rooting, improve water retention capacity and result in a more even distribution of nutrients in the soil profile. The retention of macronutrients and availability of micronutrients are enhanced by organic manuring practices. The application of highly soluble inorganic fertilisers, generally results in higher losses of macronutrients, can cause nutrient imbalances and disrupt crop uptake of all nutrients and can damage crop roots. Earthworms, which contribute significantly to soil fertility, are generally found at higher population densities in organically manured soils.
Inorganic fertilization practices which impair soil structure can limit the cycling of some crop nutrients, and accelerate the loss of others. Chemical impacts of inorganic fertilization tend to enhance nutrient losses from the soil, whereas organic manuring generally promotes a more efficient cycling. The slower nutrient release rate of organic manures, while promoting more efficient cycling and use, can limit availability to the crop. Greater emphasis is placed on achieving efficient nutrient cycling in organic farming systems. The balancing of crop and livestock enterprises, and attention to waste handling and management on organic farms, reflects this.
Nutrient cycling efficiencies within farming systems have important implications for water resources. Addition of phosphorus to surface waters causes eutrophication. Losses of phosphorus tend to be larger from conventional than organic systems, due to soil erosion effects and disposal or accidental loss of livestock wastes. Nitrate contamination of groundwaters in many areas is closely linked to agricultural practices, but the relative impacts of conventional and organic systems are difficult to evaluate. However, the application of large quantities of inorganic nitrogen fertilisers in conventional systems generally leads to a greater availability, and therefore potential for loss, of nitrogen, than do the organic and biological sources used in organic systems.
The organic approach of maximising nutrient cycling is more conserving of non-renewable energy and mineral resources than is the conventional approach based on linear throughputs of nutrients. However, the potential for enhancing nutrient cycling between the human population and agriculture as a whole is seriously limited by infrastructural and technical considerations. Recommendations for research are made, to promote a more sustainable and resource efficient approach to the maintenance of fertility in agricultural soils.
Fertilisation in Conventional and Organic Farming Systems
Fertiliser Practice and the Soil Environment
A. Physical effects relating to soil ecology
B. Chemical effects relating to soil ecology
(ii) Salt concentrations in the soil
C. Biological effects relating to soil ecology
(ii) Effects on soil microflora
(iii) Pesticide impacts on soil fauna and flora
A. Physical effects relating to soil fertility
(i) Soil structure and fertility
(ii) Water retention and crop growth
(iii) Distribution of soil nutrients in the soil profile
B. Chemical effects relating to soil fertility
(ii) Chelates and the provision of micronutrients
(iii) Carbon : nitrogen ratios
(iv) Nutrient form and crop damage
(v) Effects of fertiliser practice on nutrient balances al uptake
C. Biological effects relating to soil fertility
(i) Soil fauna and soil fertility
(ii) Soil microflora and soil fertility
A. Physical effects relating to nutrient cycles
B. Chemical effects relating to nutrient cycles
C. Biological effects relating to nutrient cycles
(i) Nutrient availability, efficiency of uptake and leaching
(ii) The influence of agriculture system design on nutrient cycles
The Wider Environmental Effects of Fertiliser Practice
1. Nutrient Losses by Leaching
A. Surface water nutrient enrichment: eutrophication
B. Groundwater nutrient enrichment
2. Nutrient Losses by Volatilization and Denitrification
The first part of this report (Arden-Clarke & Hodges, 1987) considered the impact of the development of intensive conventional farming systems on the structure and integrity of agricultural soils and on the developing problem of soil erosion, with particular reference to the position in Britain. This second part is primarily concerned with the different approaches of conventional and organic farmers to the problem of supplying sufficient plant nutrients to their growing crops, i.e. fertilization.
Whereas many of the interactions between conventional agriculture and the environment have been described elsewhere, this review attempts to interrelate and compare the often widely differing impacts of conventional and organic systems upon the soil and its fertility; an approach which has apparently not been attempted before. Consequently, many aspects are considered at greater length than might otherwise be appropriate, and there may also be a certain amount of overlap between sections.
The development of modern conventional farming has taken place over about 40 years, and a great deal of research has been undertaken into its development and its inter-relationships with the soil and the environment. On the other hand, organic farming is only at an early stage of development with comparatively little directly related research having been undertaken; there is often only a relatively limited understanding of the biological mechanisms that the organic farmer is attempting to harness in order to maintain soil fertility and control crop pests. As there are also currently relatively few working organic farms, this paper is more concerned with the potential of organic farming methods to minimise certain negative environmental impacts in the future, than with its impacts at present.
Varying quantities of the following chemical elements are necessary for the unimpaired growth of crop plants (Mengel & Kirkby, 1982; Marschner, 1986): nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulphur (S) and chlorine (Clothe macronutrients; iron (Fe), manganese (Mn), zinc (Zn), boron (B), copper (Cu), molybdenum (Mo) and cobalt (Co~the micronutrients or trace elements; and sodium (Na), silicon (Si) and cobalt (Co)the micronutrient/"beneficial" elements (Marschner, 1986). These elements will hereafter be referred to by these symbols. Macronutrients are required by crops in amounts ranging from a few kilogrammes per hectare (kg/ha) to a few hundred kg/ha, whereas micronutrients are needed in quantities ranging from only a few g/ha to several hundred g/ha (Cooke, 1982). N. P and K are usually the critical elements in terms of limitation of crop yields in Europe, and are those most commonly incorporated in synthetically compounded, "commercial" fertilizers. On many soils in Scotland S is in limited supply, and the available Cu and Mn have to be supplemented in some places. Additions of at least some of the nutrients listed above are necessary where there are net losses from agricultural ecosystems, consequent upon export in crops and their inadvertent loss by processes such as leaching, soil erosion and volatilization. Recycling of these nutrients can never be 100% efficient, even if all human wastes were returned to the land, as losses from the system are inevitable at some points in the cycle. Where these losses are not balanced by natural chemical weathering of the bedrock, or gains from other natural sources such as rainfall, some import of plant nutrients is necessary if crop yields are not to be limited by nutrient supplies. Where conventional and organic farming systems differ is in the form in which these nutrients are supplied to the soil, the organic carbon content of the fertilisers used and in the emphasis that is placed on retrieval and recycling of nutrients as opposed to importation to replace losses. The chemical form of the supplied nutrients and the organic carbon (C) content of the fertilisers have far-reaching implications for the ecology of the soil, while the relative proportions of nutrients lost and recycled have implications for neighbouring ecosystems and certain finite resources.
The different approaches to the problem of plant nutrient supply in conventional and organic farming systems is as much a product of conceptual differences in attitude towards the soil and the agricultural ecosystem, as of differences in materials, methods and techniques. Returning to the USDA definition of organic farming quoted in part I, we find that ". . . the concept of the soil as a living system . . " (USDA, 1980), is an integral part of the organic farmers approach to crop production. Organic farmers rely upon crop rotations, various organic manures, mechanical cultivation, mineral-bearing rocks and aspects of biological control to maintain soil productivity and filth, supply plant nutrients and control insects, weeds and other pests (USDA, 1980). Fertiliser regimes in organic farming systems are designed to feed the soil, such that natural nutrient cycles are enhanced and disruption of the complex biological processes on which these cycles depend is avoided (Hodges, 1978; 1981). In contrast, fertilization practices in conventional farming systems aim to deliver plant nutrients directly to the crop in simple chemical form. Though the importance of the microbial component of the soil ecosystem in nutrient retention and cycling has been recognised, far less emphasis is placed on the biological properties of the soil. In comparison to the approach of organic farmers, many conventional farmers tend to treat the soil as a relatively inert matrix accommodating the roots of the crop and retaining a proportion of the applied nutrients until such time as they are taken up by those roots. On organic farms emphasis is placed on a recycling of plant nutrients within the system, whereas on conventional farms, nutrient flow takes the form of a linear throughput rather than a cycle. As a consequence, much of the plant nutrient supplying media used on organic farms is derived from organic wastes generated on that farm, whereas many conventional farms are almost wholly dependent on external (off-farm) sources of plant nutrients.
A combination of organic materials, relatively insoluble inorganic materials and certain crops (known as green manures) supply the necessary plant nutrients on organic farms. Organic wastes applied include crop residues, animal manures and off-farm wastes; the inorganic materials are generally mineral-bearing rocks (e.g. rock phosphate) and the green manures are generally leguminous plants (e.g. clover and lucerne) which have the ability to fix atmospheric nitrogen. On most conventional farms the majority of the plant nutrients applied are in the form of inorganic fertilizers, simple chemical compounds, synthesized in a factory or obtained by mining, which are generally highly soluble in water, and which usually contain significant amounts of only a few macronutrients. There is also limited recycling of crop residues and possibly animal manures, ensuring that a proportion of the contained nutrients remain in the system. The practice of including a grass break in arable rotations has been retained by many conventional farmers, and in some areas, e.g. much of Scotland, crop rotations and the utilization of organic manures are retained as an important part of the farming system. On the other hand, in many areas of eastern England, e.g. East Anglia, conventional farms are frequently purely arable operations running a simple rotation of cereals interrupted by occasional break crops.
For the purposes of this discussion a formal distinction is made between inorganic fertilisers (defined above) and organic manures, defined by Cooke (1982) as being composed mainly of wastes and residues from plant and animal life which contain much carbon and relatively small percentages of plant nutrients. This definition covers animal manures, crop residues, green manures and various off-farm organic wastes such as sewage sludge. Cooke (1982) recognised a third category, organic fertilizers, which he identified as usually being wastes from industrial processing of plants or animals. Examples are bonemeal, leather waste, hoof and horn, and dried blood. These contain more N and P than organic manures but are generally used in much smaller quantities than organic manures and therefore supply only a small proportion of the applied nutrients. There are one or two types of soil amendment which do not fit readily into any of these categories. Synthetically compounded urea is one, an organic compound derived from an industrial process, but which also occurs naturally in the urine of livestock. Synthesized urea is no longer used on organic farms (B.O.S.C., 1985), but is a major source of N on conventional farms. Organic farmers sometimes use a soil amendment based on calcified seaweed, the main object of this practice being to improve quality and palatability of crops and grass (Vine & Bateman, 1981). This paper is primarily concerned with the contrasting effects of inorganic fertilisers and organic manures and other aspects of nutrient supply and cycles in conventional and organic farming systems.
The differences between the plant nutrient supplying media (for which fertiliser is used as a general term) used in the two types of farming system result in widely contrasting effects on the physical, chemical and biological components of the soil environment, especially when allied to other differences in methods and technologies deployed in the different farming systems. Unfortunately, our understanding of undisturbed soil ecosystems is limited, largely due to their complexity. Treating soil ecology, soil fertility and the cycling of nutrients through the soil ecosystem separately would not fully reflect this complexity. However, these processes/phenomena have had to be used as subject headings to provide a structure for the review. The physical, chemical and biological effects of fertilization practices on soil fertility, soil ecology and nutrient cycles are described under sub-headings. Again, this is not a true reflection of the complex interlinkage of these processes and effects. Wherever possible, these links are emphasized in the text, and it is hoped that this will be seen as a satisfactory approach to an extremely complex subject.
The widespread and continuing increase in the use of inorganic fertilisers may be explained by the fact that they do offer some major advantages over organic manures, mainly in terms of convenience and economy. Briggs & Courtney (1985) listed the following benefits of inorganic fertilizers: they are cheap, clean, easy to handle, store and transport, of a guaranteed composition, facilitate even and accurate application and can often be applied during other agricultural operations (such as tilling or sowing), thus reducing the number of passes necessary over the land. They are also associated with higher, more predictable yields (though the improvement in yields over established organic farms may only be 10-20~o for some crops, e.g. cereals), which are currently regarded as sustainable. In contrast, though most organic manures are effectively free (in mixed farming systems), they tend to be malodorous, are bulky with relatively low plant nutrient contents and so are less easy to handle, store and transport and are of a highly variable nutrient composition. Application of solid organic manures is less even, and that of all organic manures generally requires an additional pass over the land. Briggs & Courtney also list some of the beneficial properties of organic manures that are not shared by inorganic fertilizers. These are the high organic matter content, which has a beneficial effect on soil structure, long-term residual effects and prevention of leaching. The present account seeks to expand on this rather superficial comparative assessment (Briggs & Courtney were primarily interested in conventional agro-ecosystems) by examining the very different effects of these fertiliser practices on the soil environment, in the context of the different farming systems. Without such a comparison it is not possible to fully appreciate the far-reaching agricultural and environmental effects of the recent switch (essentially post-1940's) from the use of organic manures to inorganic fertilisers.
The complex physical, chemical and biological interactions affecting soil ecology, fertility and nutrient cycles, which are themselves intimately related, make it difficult to compile a coherent overview. Each component of each of these soil characteristics or processes is reviewed in turn below, but it is crucial to bear in mind the complex interactions within the soil ecosystem. The review will highlight these links as far as possible, at least identifying causal factors and corresponding effects. Under the heading of soil ecology, the physical, chemical and biological effects of the contrasting fertiliser regimes on all the living components of the soil ecosystem except the crop, will be considered. The section entitled soil fertility details the direct effects of different fertilisers on the ability of the soil to yield a healthy crop and also the indirect effects, borne of the effects on the other living components of the soil ecosystem, detailed in the previous section. Nutrient cycles are of course an integral part of soil fertility but the section on nutrient cycles is primarily concerned with the flow of essential plant nutrients, though this will be related to soil ecology and fertility. Throughout the review attention will be focussed mainly on nitrogen, phosphorus and potassium, given that they are needed in the greatest quantities by plants and are most commonly the elements that limit agricultural productivity.
Fertiliser practice has profound effects on the physical structure of the soil, as outlined in part I of this report (Arden-Clarke & Hodges, 1987). Rather than repeat that part of the report, a few salient points relevant to a discussion of soil ecology, fertility and nutrient cycles will be referred to and elaborated upon. It is clear that fertilization practices in organic farming systems tend to raise or maintain soil organic matter levels whereas conventional farming practices, particularly in intensive arable systems, tend to lower those levels. It should be noted, however, that even regular dressings of a single type of organic manure, such as farmyard manure (FYM, a mixture of straw and animal wastes), will not raise soil organic matter (SOM) levels dramatically, and may not even maintain them if the land is continuously under arable crops (e.g. Eagle, 1975; Russell, 1977). On an individual basis, grass or grass/clover leys are more effective in this respect, but the combination of the two is most effective. In organic farming systems, where SOM levels can be raised sharply, this is a consequence of a wide range of organic manuring techniques (leys in rotation with arable crops, FYM, crop residue and off-farm organic waste applications, and green manuring), rather than any one organic input. The physical effects of raising SOM levels are now well established (see for example, Kononova, 1966; Koepf et al., 1976; M.A.F.F., 1976; Chancy & Swift, 1984) and are summarised by Russell (1977) as: reducing the bulk density of the soil and increasing its water holding capacity; increasing the porosity of soil clods thus making them easier to shatter (i.e. more tillable) and increasing their resistance to slaking, thus enhancing the stability of the soil structure; reducing the vulnerability of the soil to mechanical damage by tillage implements in wet conditions and generally improving soil drainage in wet conditions.
The size of the soil invertebrate population depends on the physical conditions of the soil (as well as the food supply), these animals requiring a fairly well aerated and drained soil which is not compacted, i.e. with a low bulk density (Russell, 1973). The development of anaerobic conditions is more likely to occur in poorly drained, poorly structured soils (AAC, 1970), especially when cultivation has taken place in wet conditions (see "Untimeliness of Cultivation", in part I). Anaerobic conditions, noted by the AAC (the Strutt Report) as a common occurrence on conventional farms, can severely affect crop yields by restricting germination and limiting root development, and also has deleterious effects on the soil flora and fauna. Soil structure may deteriorate further under these conditions (AAC, 1970). Waterlogging of agricultural soils also tends to have detrimental effects on soil flora and fauna (e.g. Edwards & Lofty, 1977). One of the main recommendations of the Strutt report was that drainage be improved, and this has now been widely undertaken. Anaerobic conditions are by no means exclusively associated with inorganic fertilization and conventional management techniques. Some poorly structured soils are inherently prone to establishment of these conditions and they can be created by excessive applications of organic manures. This would be a rare event on organic farms where the supply of organic manures is unlikely ever to exceed adequate levels.
Fertilising soils with inorganic fertilisers as opposed to organic manures, causes major changes in the soil chemistry due to the input of large amounts of N. P and K. These chemical effects directly influence the availability and cycling of nutrients, and also impinge on the soil flora and fauna, thereby mediating an additional influence on soil fertility and nutrient cycles. In this section these direct effects on soil flora and fauna are examined, the consequences being detailed in later sections.
Application of inorganic fertilisers is likely to cause wider fluctuations in soil pH than do organic manures. This is a consequence of both the more chemically reactive nature of inorganic fertilisers, and the lowering of organic matter levels which often accompanies inorganic fertiliser practice in conventional agriculture. The former effect is the more direct and tends to be the more serious whereas the latter is rather the result of attrition of homeostatic mechanisms within the soil environment.
Inorganic N fertilisers without a metallic cation deplete reserves of base elements in the soil, thus making it more acid (Cooke, 1967). All ammonium fertilisers have this effect, causing a displacement of exchangeable calcium from soil colloids. The calcium is 'leached' from the soil in percolating water in the company of a mobile anion. The more mobile the anion, the greater will be the losses of calcium and the lowering of pH. Sulphate and chloride are such anions, and ammonium sulphate can have a particularly marked effect on soil pH levels; application of 100 kg of ammonium sulphate causes a calcium loss equivalent to about 100 kg of calcium carbonate (Cooke, 1967). When the calcium or other leached bases are not replaced (by liming, for example), a hydrogen ion takes their place and the soil becomes more acid. Ammonium phosphate does not have such a severe effect as the phosphate anion is fixed by the soil. Substitution of nitrate fertilisers for ammonium salts can also alleviate the problem as nitrates tend to be rapidly taken up by the crop, in which case no bases are leached from the soil. Including a source of calcium with the nitrogen fertiliser (e.g. ammonium nitrate mixed with limestone, "Nitro-Chalk") can alleviate this problem. It should be noted that some calcium is lost naturally in exports of farm produce, a cereal crop removing 10-20 kg Ca/ha, a kale crop up to 150 kg Ca/ha. However, in practice these losses are not nearly as significant as those from leaching or by soil acidification due to fertilisers (Cooke, 1982).
Inorganic phosphate and potassium fertilisers do not have such profound effects on soil pH. Though superphosphate has an acid reaction it does not appear to acidify the soil while basic slag (a less soluble source of P often used in organic farming) provides calcium equivalent to two-thirds of its weight in calcium carbonate (Cooke, 1982). Potassium fertilisers generally do not have large effects either, though high application rates of the chloride salt (KC1) result in leaching of the chloride ion with which a cation must be lost too, generally calcium. Calcium losses may be high, but if there is no net loss of bases (K substituting for Ca), the soil will not become more acid. In contrast, most organic manures including FYM, do not have an acidifying effect on the soil solution, the only exception being poultry manure which has a high N and low calcium content and therefore tends to acidify the soil (Cooke, 1982). In addition to providing calcium (and the whole range of micronutrients in the case of FYM - Cooke, 1976), organic manures can have an important role in increasing the buffering capacity of soils, preventing marked fluctuations in pH. Most of the soil's buffering capacity resides in the humus and clays, which hold acid and basic cations. The more humus' present, the greater is the buffering capacity (Allison, 1973). Thus organic manuring practices which raise SOM levels will enhance the soil's buffering capacity. This is reflected in the findings of Stroo & Alexander (1986), who showed that an increase in soil organic matter levels decreased the magnitude and duration of acid rain induced changes in the pH of the soil solution.
Current fertiliser trends relevant to soil acidification in the UK can be summarised as a continuing increase in application rates of inorganic N fertiliser (e.g. Church, 1985), a fall in the use of organic manures and probably a continuing decline in the use of lime. The proportion of ammonium sulphate (the most acidifying of the N fertilisers) applied is now small, but more total N fertiliser is now applied. In 1969, inorganic N was being applied to UK arable and grass crops at an average rate of 72 kg/ha, in 1974 at 89 kg/ha (Church, 1975), in 1980 at 120 kg/ha and at 147 kg/ha in 1984 (Church, 1985). Application rates have therefore doubled in the last fifteen years and the rising trend continues. More chloride is also being supplied as potassium salts and less calcium is supplied in superphosphate which has been substituted by ammonium phosphate. The Strutt Report (AAC, 1970) noted the decline in both organic manuring and liming practices and issued a specific warning on the dangers of a continued reduction in the liming rate. However, both trends have continued (see part I with reference to organic manuring). The decline in liming rates may well have been further exacerbated by the withdrawal of the government subsidy on liming materials in 1976. Johnston & Winham (1980) calculated that the minimum rate of loss of calcium from soils due to all factors, was equal to or greater than the liming rate over the previous ten years. Unless the situation is rectified, there is a serious risk that soils in some areas will become too acid and crop yields will suffer (Cooke, 1982).
The highly soluble salts which constitute inorganic fertilisers can have a considerable impact on soil chemistry. While some ions (e.g. ammonium, potassium and phosphate) bind to soil constituents, others (such as nitrate, chloride and sulphate) remain in the soil solution raising its osmotic pressure (Cooke, 1967). This can interfere with water uptake by the crops or even damage the roots and germinating seedlings. These and other effects are discussed in the section on chemical effects relating to soil fertility. High salt concentrations affect other components of the soil ecosystem such as microorganisms and invertebrate fauna. However there appear to be few published data on this subject. Suffice to say that Marshall (1977) cites a few authors who record a reduction in faunal species composition and diversity with increasing salt content and, more specifically, injury due to osmotic stress in certain species such as nematodes.
The differing fertiliser regimes of conventional and organic farming systems have both direct and indirect effects on the soil biota. Direct effects relate to the nature of the soil amendments used (e.g organic matter content and other chemical constituents), whereas the indirect effects are mediated by some of the physical and chemical changes mentioned above. As the majority of the soil flora is heterotrophic, food supply in the form of organic matter exerts the most profound biological effect on flora and fauna. This section will begin by examining the biological effects of inorganic fertilisers and organic wastes used as manures and will close with a review of the effects of particular crops which are used as fertility builders in organic systems (e.g. clover/grass leys and green manures).
Organic matter returns to the soil consequent upon differing fertiliser regimes are far and away the most important determining factor governing effects on the biological component of the soil ecosystem. Unfortunately, though some very valuable data have been collected on the contrasting effects of inorganic NPK fertilisers and FYM on SOM levels, virtually all the experiments have been carried out in the context of conventional agricultural systems. Organic farming systems employ a range of complementary organic manuring techniques to maintain or raise SOM levels. There exists no direct comparison of SOM levels maintained in experimentally comparable conventional and organic farming systems. Thus, as was the case in part I of this report, it is necessary to rely largely on extrapolation of the effects of organic manuring techniques applied singly in conventional farming systems. This makes it difficult to reach a precise comparison of SOM levels maintained by the differing farming systems.
Under the auspices of the Rothamsted Experimental Station a series of classical experiments have been carried out at a number of sites in southern England, mostly at Rothamsted and Woburn, comparing the effects of different fertiliser practice on a number of soil parameters. The results of these experiments will be referred to frequently in later sections. Here, data are quoted on the effects of inorganic fertilizers, FYM and green manures on SOM levels. In part I reference was made to increases in SOM brought about by the use of rotational leys and the return of crop residues to the soil. These references will not be repeated--suffice it to say that the general consensus is that short- to medium-term leys are usually the most effective method for raising SOM levels in quantitative terms, and that return of crop residues can also yield significant increases.
Comparative experiments reported by Mattingly (1974) showed that applications of FYM to continuous cereal crops at a rate of nearly 18 t/ha/y for 50 years resulted in organic carbon levels in the soil nearly 10% above the original level (1.49% C to 1.63% C) 23 years after this practice ceased, a result indicative of the enduring residual effects of some organic manures. By comparison, applications of inorganic fertiliser resulted in a 45% fall in soil organic carbon levels (1.49% C to 0.82% C) under continuous wheat, and a fall of 57% (1.49% C to 0.64% C) under barley. Jenkinson & Johnston (1977) found that plots receiving FYM at the high application rate of 35 t/ha/y between 1852 and 1975, had trebled soil organic C contents, whereas the inorganically fertilised plots showed little change in organic C levels. Low levels of FYM application do not, however, appear to have much effect on SOM levels. Draycott et al. (1977) concluded that dressings of only 25 t/ha every five years produced no long term gain in SOM levels. The contribution of green manures to SOM levels is generally not as high as that of FYM or 'other bulky organic wastes (see, for example, Woodward & Burge, 1982; Parsons, 1984; Barney, 1987; McRae & Mehuys, 1987), but there is evidence to suggest that they can at least maintain SOM levels under conditions where soil under crops given only inorganic fertilisers suffers a reduction in these levels (Mattingly, 1974). Dyke et al. (1977) suggest that the reduction in SOM levels in conventionally farmed soils noted in the Strutt Report (AAC, 1970) could be redressed by undersowing cereal crops with green manures.
Though there is considerable variation in the ability of different soils, under different crops, to accumulate organic C, it is probably safe to say that when used alone rotational leys and moderate dressings (15-20 t/ha/y) of FYM can raise levels while return of crop residues and green manuring can at least stabilise soil organic C at levels above those in soils receiving only inorganic fertilizers. Application of other bulky, organic, non-agricultural wastes such as sewage sludge is also likely to have a beneficial effect on SOM levels, though this has yet to be quantified. With respect to the comparison of fertiliser practices in conventional and organic farming systems it must be stressed that many specialized, conventional farmers employ none of these techniques (or perhaps only one, e.g. application of FYM), whereas organic farmers tend to use all of them. Consequently similar soils farmed conventionally and organically are bound to have substantially higher SOM levels under organic management.
Soil organic matter constitutes the food supply of the majority of soil organisms, so that SOM levels have a profound effect on these organisms' population sizes (e.g. Edwards, 1984). Russell (1973) states that the size of the soil populations of (heterotrophic) soil organisms is controlled by the rate at which energy-containing material synthesized by plants (this term covers both crop residues and the wastes of herbivorous animals) is added to the soil.
Organic manures make a direct contribution to SOM levels in the form of dead and decaying plant material or animal wastes. Inorganic fertilisers can make an indirect contribution, where crop residues are returned to the soil, by virtue of the increase they cause in overall dry matter yields. However, several inorganic fertilisers have deleterious effects on some elements of the soil fauna, particularly when applied at high rates (Edwards, 1984).
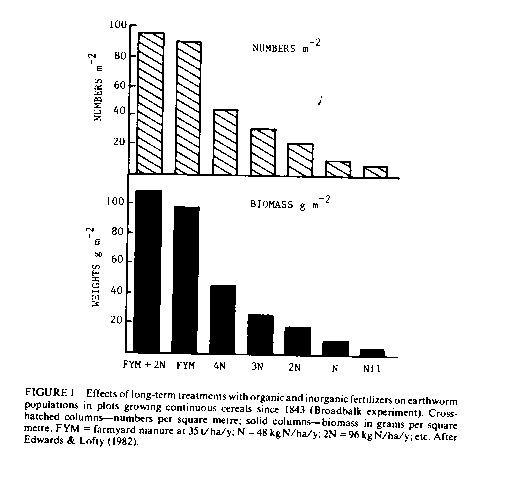
Earthworms are probably the most important soil animal in healthy agricultural soils, in terms of both their biomass and their effect on soil fertility and nutrient cycles (see below). Biomass densities as high as 1100 kg/ha have been recorded in pasture soils, though population levels in arable soils tend to be much lower, sometimes falling to 100 kg/ha. However, application of FYM to arable land at high rates (35 t/ha/y, not used in practice) can raise earthworm population densities almost to the levels recorded in pasture soils (e.g. 900 kg/ha, Russell, 1973). Edwards & Lofty (1977 & 1982) have made detailed studies of the effect of differing fertiliser regimes on earthworm populations. In a comparison of unmanured old arable plots with plots permanently under wheat receiving 35 t/ha/y of FYM, they found earthworm populations three to four times higher in the manured plots (Edwards & Lofty, 1977). Under grass, the same experimental design led to earthworm populations on manured plots three times higher than those on unmanured plots. The effects of inorganic fertilisers appear to be more variable. The evidence reviewed by Edwards & Lofty (1977) suggested that inorganic N fertilisers generally increased earthworm numbers under arable land but occasionally reduced them under grass. One inorganic N fertiliser was invariably antagonistic to earthworm populations, namely ammonium sulphate, presumably due to its acidifying effect on the soil. Applications of ammonium sulphate to hay meadows, over a period of 86 years, reduced biological activity under the pasture, leading to the build up of an undecomposed mat of organic matter (Shier & Rimmer, 1984). The organic matter accumulation impeded drainage, causing waterlogging, leading to further acidification and inhibition of organic matter decomposition. Other inorganic fertilisers are generally far less harmful to earthworms. Edwards & Lofty (1977) concluded that superphosphate has variable effects dependent on soil conditions, while other inorganic fertilisers appear to have little effect.
In more recently reported experiments, Edwards & Lofty (1982) have made more exact quantitative comparisons of the effects of FYM, inorganic NPKMg fertilsers (at various rates of N application) and mixtures of the two. The experiments were carried out on continuous cereal plots at Rothamsted and the results, illustrated in Figure 1, indicated that the mixture resulted in the largest populations of earthworms, though applications of FYM alone resulted in population densities at least twice those in plots receiving the highest rate of inorganic fertiliser alone. There was also evidence that in terms of the amount of N applied per hectare, organic matter N was more effective in raising earthworm populations than inorganic N. Applications of sewage cake had an even greater beneficial effect on earthworm populations than FYM, but large doses of sewage sludge did not result in large increases in earthworm populations. More recent observations by Scullion & Ramshaw (1987) indicate that organic manuring practices may have greater effects on behavioural patterns of earthworms than on their population size. These authors showed that applications of poultry manure encouraged casting and burrowing to the surface, whereas applications of inorganic fertiliser discouraged these activities, especially at high application rates. Scullion and Ramshaw's (1987) results also indicated that inorganic fertiliser led to significantly lower population densities of the two species "primarily" responsible for surface casting (Lumbricus terrestris and Allobophora caliginosa). Whatever the precise cause, reductions in earthworms, surface casting and burrowing activities will restrict their ability to contribute to soil fertility and nutrient cycling (see section 2,C).
Edwards & Lofty (1969) reported the results of similar experiments investigating fertiliser effects on springtails (Collembola) and mites (Acarina), numerically important members of the soil arthropod community. The effects of inorganic fertilisers and FYM on these groups were similar to those on earthworms, highest population densities being recorded on FYM manured plots, intermediate densities on NPK fertilised plots and lowest densities on unfertilized plots. Detailed comparative assessments do not appear to have been made for other faunal groups. Marshall (1977), reviewing the available data, found that though both organic manures and inorganic fertilisers tended to increase populations of other insects, myriapods (millipedes and centipedes) and enchytraeid worms, some of these species were sensitive to some inorganic fertilizers. Explanation of these quantitative differences in the effects of organic manures and inorganic fertilisers on soil fauna is by no means simple, given the wide range of differing effects of the two types of fertiliser. However, it seems likely that the generally more beneficial effect of organic manures is due to a combination of a greater increase in SOM levels and possibly less disruption of the chemical environment of soil animals. Edwards & Lofty (1969) showed that soil pH was having a considerable influence on springtails and mite populations, those in limed plots being larger. It is well known that soil pH has a profound influence on earthworm activities, these animals generally preferring soils with a pH of about 7.0 (Edwards & Lofty, 1977), while most species are unable to survive at a pH of 4.5 or less (Russell, 1973).
It should be noted that excessive applications of some types of organic manure can also have deleterious effects on soil fauna. For example, Curry (1976) found that high rates of liquid slurry application (562,625 1/ha/y) had severe consequences for earthworm populations, numbers being reduced by over a half and biomass by over a third. However, such practices are almost invariably a consequence of the need to dispose of the large excesses of animal waste generated by intensive livestock husbandry units which are not a feature of organic farming systems.
Comparisons of the effects of green manuring and crop residue incorporation on soil fauna cannot be made as there are no adequate published data. It would seem likely, though, that they have effects similar to those of other organic manures in qualitative terms, but lower in quantitative terms.
Fertiliser practice directly affects soil populations of fungi, bacteria and viruses by virtue of its effects on the physical and chemical conditions of the soil. Indirect affects will also accrue, due to effects on the living components of the soil environment, on which the soil flora and micro-organisms depend for food and other resources. This complex of inter-relationships is poorly understood--in fact, it is generally only those elements of microflora and viral communities which are of economic significance to agriculture, that have been subjected to detailed investigation. Consequently, the remainder of this section will dwell upon the effects of differing fertiliser practices on soil-borne diseases and pathogens.
The value of plant residues and other organic amendments in attaining biological control of soil-borne plant pathogens is well established. Though there may occasionally be detrimental effects on crop roots due to phytotoxins produced during the decay of organic residues (Elliott & Papendick, 1986), there is no doubt that the beneficial effects in terms of disease control, far outweigh these deleterious effects (Patrick & Tousson, 1965; Baker & Cook, 1974; Cook, 1977, 1981 & 1986). Other antagonistic effects of organic manures on plant pathogens listed by Cook (1977) include: the immobilization of soil N by organic amendments of a high C: N ratio (a phenomenon explained in the section on chemical effects relating to soil fertility, p. 246); the production of antibiotics by saprophytes; and the release of ammonia or other volatile compounds that may inhibit the development of pathogenic organisms. Allison (1973) lists a number of other possible contributory effects, namely--alteration of soil pH, increase in carbon dioxide concentration, decrease in oxygen concentration and an increase in the supply of plant nutrients which enhances host resistance. Allison felt that the production of antibiotics by saprophytes was one of the chief mechanisms of natural suppression of plant pathogens.
The beneficial effects of organic amendments in this respect are undoubtedly complex and are as yet poorly understood. Some of the assumptions made in designing conventional agricultural systems may well be challenged by the results of future work in this area. There are some interesting anomalies relevant to this form of biological control which deserve further investigation. For example, Huber & Watson (1974) noted that organic amendments may decrease disease levels even if their application raises population levels of the pathogen.
Inorganic fertilisers can reduce crop diseases in soils where they rectify nutrient imbalances which would otherwise increase the susceptibility of the host to damage and infection. Coleman & Ridgway (1983) list a number of such cases involving the supply of nitrogen, calcium and phosphorus. However, these nutrients can also be supplied by organic manures and inappropriate use of inorganic fertilisers can often lead to nutrient imbalances, rather than preventing them (Hodges & Scofield, 1983). In particular, most inorganic fertilisers do not contain any trace elements (micronutrients) and Bussler (1974a) suggests that these minor elements may be important contributory factors to stress tolerance. Deficiencies in these elements result in metabolic changes and cell damage that raise the susceptibility of the plant to pathogen infection and pest infestation.
Where inorganic fertilisers are not employed with care, the consequences for the health of crops may be serious. It is generally accepted that high application rates of nitrogenous fertilisers can increase the susceptibility to pathogens such as rusts, powdery mildews, blasts and smut fungi (Whitney, 1976). The increase in the susceptibility of cereals to leaf pathogens which is often caused by applications of N fertiliser can limit the yield potentially obtainable from the extra nitrogen (Jenkyn, 1976). The mechanism responsible for this decreased resistance has yet to be satisfactorily explained, but Kiraly (1976) noted that the reduction in plant phenols found in several studies of N fertiliser effects, could be an important factor. The same author also notes that it is generally only susceptibility to obligate parasites that is increased by inorganic N fertilization, infections due to facultative parasites usually being reduced by high rates of N application.
The effects of inorganic P and K fertilization are not as consistent nor apparently as great as those of N fertilization. Whitney (1976) claimed that though some viral infections are reinforced by applications of these fertilizers, such compounds generally increase crop resistance to pathogens. Potassium fertilization has been shown to reduce several infectious diseases, such as wheat rusts, and stimulate wound healing which also increases disease resistance (Kiraly, 1976). The same author was less sanguine about the effects of inorganic P fertilization, noting that it had no consistent effect on plant disease resistance. The acidification of the soil and leaching of calcium caused by some nitrogenous fertilisers may have a less direct adverse effect on plant resistance, as Kiraly notes that adequate calcium is important for plant cell wall resistance to degradation by pathogens. This illustrates a more general point, namely that large applications of highly soluble fertilisers (and other reactive chemical compounds) can interfere substantially with the complex processes governing plant nutrition, development and disease resistance. El-Fouly (1976) describes an illuminating sequence of side-effects and counter applications of various agrochemicals which begins with the application of inorganic N fertiliser. This can cause "lodging" in cereals, a consequence of a change in the growth form of the plant which leaves the stem too weak to support the weight of the grain and leaves. The plant collapses, wind and rain determining the severity of this effect, which makes harvesting more difficult and can even preclude it. A synthesized plant growth substance, Chlormequat (Cycocel or CCC), is applied to reduce lodging by regulating the plant's growth. However, these changes in growth wrought by Cyclocel, while reducing susceptibility to some diseases (possibly because it increases the levels of phenols in the crop), dramatically increased susceptibility to infection by the fungi Septoria and Fusarium ssp. This effect can be so severe as to nullify the positive effects of lodging prevention. In view of this effect and the fact that the eye-spot resistance achieved is only partial, the author recommends a "package treatment" combining N fertiliser, Cyclocel and systemic fungicide. Thus where there was originally only one chemical being applied, there are now three, with no guarantee that more side-effects will not develop (e.g. from the fungicide), even if high yields are obtained initially.
Grass leys and green manures have beneficial effects in terms of pest and disease control other than disease suppression linked to the raising of SOM levels. Green manures enhance weed control when undersown in cereal crops (Dyke & Barnard, 1976); the importance of crop rotations in this respect and in general disease suppression is well established. This subject will be reviewed in part III of this report, devoted to impacts of pest control strategies on the crop ecosystem. This third section of the report will also examine the effects on soil flora and fauna of some of the biocidal compounds used in conventional agriculture. The subject also deserves mention here as these effects often have implications for soil fertility and nutrient cycles. Most of the data collected to date refer to effects on soil fauna rather than flora.
The elimination of crop pests by a range of pesticides of varying degrees of selectivity, as practiced on conventional farms, is bound to have an impact on soil fauna and flora in general. Whereas traditional (and organic) farmers stimulate biological activity by organic manuring practices, a conventional farmer tends to destroy their activity by using pesticides (Fedoroff, 1987). Nematicides appear to be the most toxic of all pesticides to soil fauna (Edwards, 1984), being lethal to virtually all organisms in the soil. The fumigant varieties such as methyl bromide have the most dramatic effects. Edwards & Lofty (1971) showed that while aldicarb, methomyl and dazomet all reduced populations of earthworms, potworms and insects, dazomet had the greatest effect, particularly on the earthworm population (reduced to just over a third of the population in untreated control plots). Repeated applications of the fungicides benomyl and thiophanate-methyl, have been shown to result in drastic population reductions of a range of earthworm species (Stringer & Lyons, 1974). Populations of the surface feeding species Lumbricus terrestris and Allobophora chlorotica were virtually eliminated after two years of spraying experimental plots in orchards. The wide range of insecticides used in conventional agriculture have equally diverse effects on soil inverterbrate populations. Some are selective in their action whereas others will kill a range of species (Madge, 1981). The use of persistent insecticides (e.g. organochlorines) has been a major cause for concern in view of their long-term effects, but their use is now generally being phased out. Madge reviews the diverse effects of many of these substances on soil fauna, which defy concise summary. Suffice it to say that these compounds can disrupt arthropod predator-prey relationships and that earthworms are not generally susceptible, though some less persistent organochlorines such as dasanit may have significant detrimental effects on these animals.
Only a few herbicides are directly toxic to soil fauna, though indirect effects consequent upon their weed-killing properties which result in the elimination of an important source of decaying plant matter, can reduce the available food supply for saprophytic flora and fauna. Different herbicides affect different groups of soil fauna, but with the exception of the triazines generally do not have direct effects and do not constitute a serious danger to the soil faunal community (Madge, 1981). There are few data on the effects of fungicides applied to the soil, though presumably any non-specific formulations will have detrimental effects on beneficial soil fungi such as vesicular-arbuscular mychorrhizae if they penetrate far enough to reach them (see section on biological effects relating to soil fertility, p.250). Most fungicides do not appear to have deleterious effects on soil fauna, though some, such as benomyl, can sharply reduce earthworm populations (Wright, 1979).
In a purely agricultural sense, the fertility of a soil may be defined as its capacity to produce the crops desired (Russell, 1973). To view the soil's fertility purely in terms of crop yield is to overlook the complex soil processes involved in its development and maintenance. Russell recognised this and referred his readers to Jacks, (1963) definition of fertility as a biophysical phenomenon. This author noted that the physical work done by those organisms living in the soil (the edaphon) was the main factor governing the formation of structure associated with a fertile soil. This definition lays adequate stress on the importance of life within the soil to the maintenance of its fertility, and should always be borne in mind when discussing the impacts of various fertiliser regimes (and for that matter, agricultural systems).
The influence of soil organic matter on soil fertility is perhaps as critical as it is on the general ecology of the soil. However, its precise role in soil fertility remains poorly understood, a fact acknowledged by Russell (1977) in his review of the subject. He stated that, "...a major problem facing the agricultural research community is to quantify the effects of SOM on the complex of properties subsumed under the phrase soil fertility, so that it can help farmers develop systems which will minimise any harmful effects this lowering (of SOM levels) brings about". The reduction in SOM commonly seen in soils in conventional farming systems causes complex and often profound changes in soil fertility. Russell (1977) noted that many farmers believed that the techniques which they were applying which lowered SOM levels were lowering the fertility of the soil. Yet quantitative differences in organic matter returns and losses brought about by conventional and organic farming methods is by no means the only factor affecting soil fertility. Comparison of the effects of the range of organic manuring techniques used in organic farming systems, and the application of inorganic fertilisers practiced on conventional farms reveals major differences in: the proportions of plant nutrients supplied (to the extent that some micronutrients are not applied at all on some conventional farms); the chemical form of these plant nutrients and hence their availability to the crop plants; and effects on soil organisms with roles in soil fertility. Some of these effects are dealt with in a later section on nutrient cycles, which examines how the flow of nutrients through all components of agro-ecosystems differs under the different farming practices. In this section the primary concern is with the transfer of nutrients from soil to plant and the physical, chemical and biological effects related to differences in fertiliser practice which affect this process.
Soil structure exerts an important influence on soil fertility by virtue of its effect on crop rooting patterns, soil moisture levels and resistance to various forms of soil degradation which can result from agricultural practices. The latter effect has been considered at length in part I of the present report. Evidence was presented that indicated that where soil structure has deteriorated, short- and long-term reductions in soil fertility (as measured by crop yields) can occur as a consequence of erosion losses of SOM, nutrients (especially N and P) and the finer soil particles (e.g. clay), reductions in the depth of soil available for rooting, soil compaction, waterlogging and the development of anaerobic conditions (see, for example, AAC, 1970; Evans & Nortcliff, 1978; Boardman, 1983).
Soil structure can have a profound effect on the growth of crop roots and consequently the crop plant as a whole. Russell (1973) considers the ability of the plant to find space to grow or to force its way into the soil, is often the most important factor limiting plant growth. An adequate network of soil pores large enough to allow free entry and growth of roots is a very important growth requirement (Scott Russell, 1977).
Limitations on root density, and in particular the numbers of finer roots, can limit uptake of some plant nutrients. Wiersum (1962) showed that large, cloddy soil aggregates resulted in the development of a coarse root system and consequent decrease in P uptake, whereas N uptake was largely unaffected. This was attributed to the relative immobility of P in the soil which results in roots being able to extract this element from only a limited volume of soil in their immediate vicinity. Cornforth (1965) was also able to demonstrate a negative correlation between aggregate size and P uptake for various crops, whereas nitrate uptake was unaffected by this parameter. Coarsely rooting crops in cloddy soil are therefore likely to have yields limited by the uptake of phosphate and other relatively immobile ions. It should be noted that the effectiveness of organic manuring in improving soil structure and increasing yields is, to a considerable extent, dependent on a number of variables such as soil type and climate (e.g. temperature and rainfall). Significant crop yield increases consequent on structural improvements wrought by organic manuring tend to be manifest only on lighter soils with higher silt and sand fractions (Cooke, 1967).
A well-structured soil has neither over-large aggregates, as described above, nor a complete absence of aggregates, as this would result in a compacted, relatively non-porous and structureless soil. It would appear that under some conditions inorganic fertilisers can cause disaggregation of soil aggregates important in the maintenance of soil structure. Wilson & Cooke (1980) listed this effect as a possible cause of increased wind erosion in the UK. Harris et al. (1966) listed five published cases where application of inorganic fertilisers to soils, in conjunction with organic manures, reduced the effectiveness of the organic manures in increasing aggregate formation. It appears that the inorganic fertilisers mediated this effect by hastening the destruction of aggregates formed, and less directly, by reducing C: N ratios in the soil thus favouring rapid decomposition of microbially synthesized soil-binding materials. It is interesting to note, however, that this property of inorganic fertilisers has been used to good effect on occasion--ammonium sulphate has been used as a soil conditioner on cloddy soils (Atkinson, 1986).
In dry years, the yields of unirrigated crops will often be limited by the water supplied by the soil. Organic matter levels in many soils appear to be an important factor determining available water capacity. Salter & Williams (1963) showed that large applications (50t/ha/y) of FYM to a sandy loam increased available water capacity in the top 15 cm of soil by up to 70%, and that this effect persisted throughout most of the field life of the crop. The organic manuring also led to an increase in the volume of water released at low tensions (namely 0.05, 0.10 and 0.20 atmospheres). It has been shown elsewhere that tension is the property of soil water most directly related to the work plants must do to extract it. This initial work was followed up by the same authors, who tested the effect of FYM applications on available water capacities of six different soils at three different sites (Salter & Williams, 1969). In all except one of the soils treated with FYM, available water capacities were significantly higher, while the volume of water released at low tensions (0.05 atmospheres) was greater in all soils. It was also noted that the available water capacity of soils recently ploughed out of ley was significantly higher than in the same soils under continuous arable cropping, and that the extra available water was held at a lower tension.
The beneficial effects of grass crops on available water capacity received confirmation from the finding of Williams (1978) that soils under old pasture had larger water-holding capacities than similar soils under arable cropping or fallow fields. Williams also demonstrated a particularly close relationship between SOM levels in loamy sands and the water-holding capacity of that soil. There appear to be no similar data for the effect of green manures on available water capacity, although this is touched on briefly by Allison (1973), but Unger (1978) has shown that application of crop residues (in this case wheat straw) at rates of 12t/ha can raise available soil water to levels about twice those of the same soil receiving no crop residues. On the basis of the above findings it would seem reasonable to conclude that organically farmed (sandy or light) soils will have higher water-holding capacities than similar conventionally farmed soils.
Organic manuring and inorganic fertilising tend to result in rather different vertical distributions of nutrients in the soil profile, by virtue of the method of application, and probably also secondary effects relating to the soil biota (see section on biological effects relating to soil fertility, p. 250). The responses of the crop to these different distributions can have important implications for crop yields.
Where inorganic fertilisers are simply spread over the soil surface, less soluble plant nutrients will tend to be concentrated in the top few centimetres of soil. In contrast, repeated applications of organic manures, made year after year, tend to raise nutrient levels throughout the soil profile (Webber, 1975). As it is well established that roots proliferate where they come into contact with rich supplies of nutrients (e.g. Weaver, 1926; Cooke, 1954; Russell, 1973; Scott Russell, 1977), this can have important implications for crops, especially in dry seasons (shallow rooting plants are more prone to the effects of drought). It has also been suggested by a number of workers that the evenly distributed and readily available supply of nutrients created by regular organic manuring practices is very important in stimulating early growth in crops (Webber, 1975).
While SOM levels have important chemical effects relating to soil fertility, the highly soluble and reactive nature of inorganic fertilisers means that their absence or presence and concentration in the soil can have equally important effects As ever, the chemical effects of variations in SOM levels are made complex by the interaction of the chemical and biological components of the soil ecosystem. In general terms, the beneficial effect of raised SOM levels on soil fertility is undoubted (e.g. Allison, 1973), but in specific terms (i.e. relating to particular crops, soils, climates, tillage practices and general agricultural systems design) it is highly variable. The differences in chemical effects of organic manuring and inorganic fertilising described below are probably more clear cut than is the case with the physical and biological effects.
The cation exchange capacity (CEC) of a soil determines the retention and availability of the various cationic macro-nutrients necessary for the production of healthy crops and maximum yields. The five macro-nutrient cations supplied by soils are calcium, potassium, magnesium, ammonium and sodium. Sodium is not essential for many crops but plays a significant and largely undefined role in the uptake of the other nutrient cations (Cooke, 1967). Ammonium is not always essential as the N requirements of a crop can be met by nitrates. The CEC (defined as the maximum number of adsorbed ions held per 100 g of dry soil or organic matter) of soils is therefore critical in regulating the supply of calcium, potassium and magnesium. The seat of most ion exchange in the soil is the finer colloidal fractions, which have both an inorganic (clay and small silt particles) and an organic (humus) fraction. These finer particles acquire a negative charge during natural soil formation processes, and it is this which leads to the binding of cations (Allison, 1973). Therefore any fertiliser practice which tends to raise SOM levels will enhance the CEC of the soil, unless that soil has a large mineral fraction with a high CELL. In that case organic manuring will only increase the CEC per unit area, but may reduce it per unit volume. The benefits of a high CEC are that the soil becomes a good "sink" for cations, consequently holding large reserves and suffering less loss by leaching of these ions (Cooke, 1967). Cations bound in this way are held with varying tenacities and are subject to replacement by other cations and removal by crop roots. It has been estimated that the organic matter content of mineral soils constitutes 30-65% of the total exchange capacity of the soil (Allison, 1973), though van Dijk(1971)suggests that this proportion may rise to 90%.
Chemical structures in which one or more organic compounds form a "claw" to surround and bind a metallic element are known as chelates. These compounds may be very important in the supply of some micronutrients (iron, manganese, zinc, copper, boron and molybdenum) to plants (Allison, 1973). In fact many of the micronutrients are so insoluble that in the absence of humus it is impossible for plants to obtain enough of them. In contrast, metal chelates are water soluble and hold the incorporated metals at varying degrees of stability (Allison, 1973). The ability of humus to hold some micronutrients in an available form is one of the most important benefits derived from high SOM levels. Micronutrients should be in greater supply in organically manured as opposed to inorganically fertilised soils not only because SOM levels are raised more by the former, but also because some organic manures contain all these micronutrients (Cooke, 1976b) whereas most inorganic fertilisers generally contain few or occasionally none. The micronutrient contents of various inorganic fertilisers and FYM are shown in Table 1, reproduced from Stojkovska & Cooke (1958). The amounts of four micronutrients supplied by commonly used dressings of some of these fertilisers are reproduced from Williams et at (1960), in Table 2.

The micronutrient requirements of various crops are also quantified in Williams et al. (1960), who found that a wheat grain crop grown with inorganic NPK fertilisers removed 20 g/ha of copper, 161 g/ha of manganese, 0.10 g/ha of molybdenum and 148 g/ha of zinc. The equivalent figures for a potato crop were 44g/ha of copper, 42g/ha of manganese, 0.74g/ha of molybdenum and 99 g/ha of zinc. Examination of Tables 1 and 2 indicates that there are clearly inorganic fertilisers, and even combinations of inorganic fertilisers, which fail to replace all the micronutrients removed by a crop, a situation which if repeated in successive years of cropping can ultimately lead to mineral deficiencies in the crops. The Strutt Report concluded that the lack of knowledge of the distribution of trace elements in soil under intensive conventional systems was a cause for concern, and actually rated this problem as more of a threat to the fertility of UK soils than soil erosion (AAC, 1970). Bussler (1974a) noted the spread of mineral deficiencies in crops grown in the US and Western Europe and linked it to the increasing use of inorganic NPK fertilisers which failed to replace soil micronutrients removed by the large crops grown under these fertiliser practices. He listed some of the morphological and histological changes and visible symptoms that arise from such deficiencies and attributed the diminution of some crops under high rates of inorganic fertilization to induced mineral deficiencies. Mortvedt (1986) links the recent increases in micronutrient deficiencies in crops throughout the world to intensification of farming practices in general and the greater use of marginal lands. The specific aspects of the intensification process causing these deficiencies are: the resultant higher crop yields increasing micronutrient demands; the use of "high-analysis", pure inorganic fertilisers resulting in lower inputs of these micronutrients; and the decreasing use of FYM and other organic manures which provide these micronutrients (Mortvedt, 1986).
The ratio of carbon to nitrogen (C: N) in the soil has a marked effect on the availability of N for crop uptake, and its vulnerability to leaching and volatilization. The higher the C: N ratio, the greater the biological immobilization of N. The ease with which a nutrient element is taken up by a plant or lost from the soil reservoir is governed by its chemical form. This in turn is affected by the antagonistic and largely microbially-mediated processes of mineralization and immobilization. Mineralization is the conversion of an organic form of an element to the inorganic state, whereas immobilization is the conversion of an inorganic nutrient element to an organic complex (Alexander, 1971). The latter is usually a consequence of assimilation by microbial cells and incorporation into the protoplasm.
In soils where organic matter (and therefore carbon) levels are low, N applied in inorganic form that is not taken up by the crop will be rapidly lost, though a proportion will be retained by microbial elements in the soil, which are usually more N limited than C limited. Incorporating an organic manure immobilizes a proportion of the mineral N in the soil, the proportion immobilized and the duration of immobilization depending on the C: N ratio. Herbert (1977) notes that no drop in soil mineral N occurs when the C: N ratio of the incorporated organic matter is below 13. However, when the ratio is between 13 and 20 N immobilization can be maintained throughout a cold winter. This has important implications for conservation of nutrient resources and pollution of the wider environment, subjects discussed later.
Applied nutrients can damage the crops whose growth they are supposed to stimulate, by virtue of changes in ionic concentrations and pH of the soil (Hodges & Scofield, 1983). The danger of such effects is generally greater with inorganic fertilisers than with organic manures, though fresh, undecomposed manures can cause damage. Damage due to high ionic concentrations tends to be rather localized (e.g. Allison, 1973) but the damage is more widespread where fertilizer practices have caused a general lowering of pH throughout the soil.
Inorganic N fertilisers are generally the most problematic fertilisers in terms of crop root and seedling damage. Imai (1977) looked at the effects of ammonium and nitrate N, in culture, on rice roots and found that harmful effects occurred at substantially lower application rates of ammonium (above 30 ppm NH4-N in culture solution) as opposed to nitrate (no toxic effects at 50 ppm NO3-N). The damage caused by the lower rates of application of N to rice and barley roots could be avoided by raising the P concentration in the culture solution or substituting some nitrate for ammonium. However, these findings are not in agreement with the assertion of Cooke (1982) that ammonium salts are generally safer than nitrates. He states that large dressings of nitrates can inhibit germination of crops like potatoes, kale and cereals if the fertiliser is incorporated into the soil in close proximity to the~crop plants. In any case there is enough evidence to suggest that highly soluble inorganic forms of N can have deleterious effects on crops.
Various other inorganic fertilisers also have damaging effects if applied at rates high enough to raise the salt concentration in the soil to the point where osmotic stress is inflicted on the crop. Very high rates of application of some organic manures, particularly liquid animal wastes, can damage crops. The Royal Commission on Environmental Pollution notes in its seventh report (RCEP, 1979) that slurry from intensive animal husbandry units applied at high rates to agricultural land (essentially as a method of disposal) has damaged crops. Such units and methods are not a feature of organic farming systems, which in any case never generate surpluses of organic wastes. Indeed, the limited supply of such wastes is a factor that will probably ultimately limit the spread of organic farming practices.
The interactions between nutrient elements in the soil are very complex, and thus the application of significant amounts of inorganic fertilisers containing only a proportion of the nutrients necessary for crop growth (usually the macronutrients N. P and K) can alter the availability of other nutrients and may lead to inhibition of the uptake of some macro- and micronutrients by crop plants (see, for example, Mulder, 1953; Schutte, 1964; Voisin, 1965; Bussler, 1974b; Davidescu, 1974). The high application rates of inorganic fertilisers associated with many intensive, conventional arable systems can have a variety of inter-linked negative effects on nutrient availability and uptake by crops. De Haan (1987) lists the following effects. Imbalances between the proportion of elements present in inorganic fertilisers and proportions of those nutrients required by plants, can result in the necessary adjustments of applications leading to overdoses of some constituents, these overdoses having negative impacts on soil fertility. Large applications of inorganic fertilisers may change soil conditions to the point that the overdose of nutrients then required for "proper" plant production may result in undesirable effects in the long-term. Application rates of inorganic fertiliser high enough to cause such problems are yet economically feasible when judged solely on plant production criteria.
Kemp (1971) was able to show that while fertilising with K and N led to high levels of K (higher than the requirement of milking cows) in pasture grass, sodium, calcium and magnesium were often at such low levels that deficiencies resulted in grazing cattle. Raising the content of any one of these nutrients in the grass with inorganic fertiliser inputs invariably resulted in a fall of one or more of the other nutrient contents. Kemp pointed out that sodium and magnesium deficiencies are known to occur frequently in cattle in Western Europe. Several years earlier, Voisin (1965) had drawn attention to the spread of Mg-deficiency disorders in European livestock, linking it to excessive applications of K fertiliser. Voisin noted a general increase in livestock disorders related to mineral deficiencies and listed the various deficiencies linked to the application of the most frequently used inorganic fertilisers (N fertilisers reduce copper in the soil, phosphoric acid reduces available zinc and K fertilisers reduce available magnesium, calcium, sodium and boron). Takahashi et al. (1977) showed similar effects in their review of the mineral composition of grass in intensively managed pastures in Japan which received heavy inputs of inorganic NPK fertilisers. In this case the resulting deficiencies were in phosphorous, boron, copper and zinc and a lowered K/(Ca+Mg) ratio was a factor in the incidence of the cattle disease grass tetany.
The importance of organic matter, lowered soil levels of which can result in a reduced availability of micronutrients, has already been noted. However, the differences in the effect of organic manures and inorganic fertilisers on soil and crop nutrient balances are still something of a mystery. Williams et al. (1960) showed that while FYM contained the essential micronutrients and inorganic NPK fertilisers did not, both these soil amendments resulted in a depressed uptake of copper, molybdenum and zinc by crops in a five-course arable rotation. A dressing of 38//ha/rotation of FYM supplied more micronutrients than removed by all the crops, but micronutrient uptake was similar after inorganic fertiliser application which did not replace micronutrient exports.
The acidification of soils attendant on the use of some inorganic N fertilisers can, if not corrected, cause nutrient imbalances by selective leaching of some elements. The loss of calcium is an important part of the acidification process itself, but the loss of magnesium which often accompanies the acidification tends to be ignored (Cooke, 1982). If liming materials which do not incorporate magnesium are used to correct such acidification, Mg deficiencies can occur in crops (the Mg content of limestones varies from 0.1- 12.0%).
Prior to the examination of the influence of soil flora and fauna on soil fertility, the significance of the natural biological variation which manifests itself in comparisons of the effects of inorganic fertilisers and organic manures should be discussed. This variation within natural systems accounts for many of the apparent inconsistencies in the experimental evidence on soil organic matter and its influence on soil ecology and fertility. Considerable variation is observed in both the effectiveness of particular organic manuring techniques in raising SOM levels and the effects on fertility of such increases as are achieved. The review by Russell (1977) provides good examples of the wide variations in increases in SOM levels brought about by the same organic manuring technique used under different conditions (e.g. differences in soils, crop rotations, cultivations and other organic manuring practices employed in conjunction).
Super-imposed on this variation, is the variation in the effectiveness of organic manuring in enhancing soil fertility. Comparisons of FYM and inorganic fertiliser application have led to different conclusions on the quantitative effects on crop yields (see review by Cooke, 1977a). Horne (1973) showed that the increase in crop yield brought about by applications of FYM varied from 0.6-8.3% at six different sites. The fact that experimentally determined yield increases due to organic manuring have been so variable, and on some occasions minimal, has led some authors to disparage the value of these fertiliser practices (e.g. Home, 1973 and Cooke, 1977a). Recommendations have even been made not to reintroduce organic manuring practices if they compromise attainment of short-term economic objectives (e.g. Cooke, 1977a). However, Newbould (1982), pointed out that the validity of this recommendation was dependent on the farmer achieving correct application of fertilizers, efficient utilization of available resources, effective pest control and preservation of a good soil structure. Furthermore, none of these experiments have looked at organic manuring practices in the context of organic farming systems, nor have they placed much emphasis on long-term objectives such as conservation of soil structure and inherent fertility. These are omissions which should be rectified before final judgement is made on the relative merits of organic manuring and inorganic fertilization.
In the section on soil ecology it was shown how differences in fertiliser practice affected the soil fauna, organic manuring techniques usually resulting in higher soil invertebrate populations. This section examines how these differences in turn affect nutrient availability and other factors influencing crop yields.
Though soil arthropods do have some effect on soil fertility it is not as significant as that of earthworms. Before looking at the latter it should be said with respect to the former that studies in natural ecosystems have shown that arthropods can have effects on mineralization rates of some elements. It appears that in general mineralization is increased by their activities, though the balance of mineralization and immobilization is influenced by these detritivores, interaction with soil micro-organisms (Seastedth & Crossley, 1984). In some cases arthropod detritivores can be responsible for large increases in soil mineral-N.
Edwards & Lofty (1977) and Stewart & Salih (1981) emphasised the beneficial influence of earthworms on soil fertility through the effects of their activities on soil structure. Their importance in the formation of soil was also mentioned in part I of this report. The burrowing activities of earthworms improve the aeration, porosity and drainage of the soil, all of which are important factors in the development of healthy and extensive crop root systems. The beneficial effects of channel provision for roots may be enhanced by the lining of the earthworm burrow with a higher proportion of available nutrients than in the surrounding soil (Edwards & Lofty, 1980). Nutrient availabilities are also considerably higher in the casts of earthworms than in the soil from which they are derived (Lunt & Jacobsen, 1944; Syers & Springett 1984), the casts are well-structured, stable aggregates which help greatly to improve soil structure. Watanabe (1975) found that Megascolecid earthworms in Japanese pastures produced up to 61 t/ha/y of casts with increased pH, total C, total N and available calcium and magnesium. Earthworm activity also results in a thorough mixing of all the soil fractions and a more even distribution of plant nutrients throughout the soil profile, encouraging deeper rooting of the crops. The importance of even nutrient distribution and deep rooting is emphasised by Deavin (1978). Under certain conditions earthworms have also been shown to influence ion transport, and they generally increase nutrient cycling rates within the soil ecosystem (Syers & Springett, 1984).
Before the involvement of certain species of soil fauna in the mineralization of nutrients was recognised, it was assumed that this process was largely the result of the activity of soil microflora (Reichle, 1977). Their role is undoubtedly a major one, but understanding of it is by no means clear, especially since the importance of the dynamic interaction between soil fauna and microflora has been recognized. With respect to an examination of the comparative effects of different fertiliser practices, the dearth of relevant data renders it impossible to do more than make some broad conjectural statements. Such data as exist suggest that the immediate effects of different fertiliser practices on microbial populations are similar to those on the soil fauna. Thus, Fraser (1984) found that in soil amended with organic manures, soil bacterial and fungal counts and dehydrogenase activity (a measure of the overall rate of microbial metabolism) were higher than those in soils supplied with inorganic fertilizers. Martyniuk & Wagner (1978) demonstrated an even closer parallel between fertiliser effects on soil microbial and faunal populations, finding that untreated plots had lower microbial populations than those treated with inorganic fertilizers, which had lower populations that plots treated with organic manures. To date, the only direct comparison of microbial populations and activity in conventionally and organically managed soils is that of Bolton et al. (1985). These authors found that levels of urease, phosphatase and dehydrogenase were consistently (three samples), significantly higher in the organically managed soil, and that microbial biomass was significantly higher in the organically managed soil in two of the three samples. The precise cause and effects (if any) of these differences were not investigated in any detail. However, it was noted that soil pH, organic carbon content and Kjeldahl N (a measure of total organic N) were significantly higher in the organically managed soil. Mineral N levels were significantly higher (approximately twice as high) in the conventionally managed soil. Unfortunately, the small sample size on which these results are based, and the lack of a control, detract from their value and general applicability.
The increases in saprophytic microflora caused by organic manuring techniques (see biological effects relating to soil ecology, p. 234) will undoubtedly be important in terms of stimulating microbial mineralization (and presumably immobilization) processes. Yet the effects of organic manure applications are likely to vary dramatically given the complex of poorly understood variables affecting soil micro-organism populations. The effects of inorganic fertiliser application are also likely to vary widely, though inasmuch as they can cause significant disruption of the soil chemical environment (see p.231 above) their effects are more likely to be deleterious to a substantial proportion of natural soil micro-organism communities. However, even if such applications reduce microbial mineralization rates, they may have little effect on crop uptake of nutrients as inorganic fertilisers provide these in readily available forms. Where such differences will be important is in determining losses from the system (e.g. by leaching, see section on nutrient cycles). In natural soil ecosystems balanced interactions between soil flora and fauna lead to controlled, continuous mineral releases which could offer advantages over rapid increases in nutrient concentration and subsequent heavy losses from the system (Reichle, 1977). Organically manured soils are likely to resemble natural systems more closely than those receiving inorganic fertilizers.
One particular element of soil microfloral communities known to have a critical influence on the uptake of nutrients, and especially phosphorous, by plants are the mycorrhizal fungi. The fungal mycelia of these organisms intimately connect the plant to the soil (Mosse, 1986). The form of the connection made by vesicular-arbuscular (V.A.) mycorrhizae, is described in some detail by Scott Russell (1977). This author also lists the evidence which indicates that these organisms can increase the rate of phosphate uptake of mycorrhizal as opposed to non-mycorrhizal roots, by as much as a factor of three (e.g. Sanders & Tinker, 1973). Tinker (1984) concludes that the large growth increases in plants generally associated with mycorrhizal infection are almost always linked to this increased P uptake, and that uptake of zinc and copper by the plant may also be improved. Not only is the condition of the plant improved by this more efficient uptake, but nutrients are conserved within the soil ecosystem and losses due to immobilization or leaching are reduced (Read et at, 1985). These mycorrhizae are affected by fertiliser practice; Gerdemann (1975) noted that, in general, additions of inorganic N. P and complete fertilisers reduce the level of infection by V.A. mycorrhizae, though this effect does vary with the soil type.
There are major qualitative and quantitative differences in the flow of nutrients through conventional and organic agro-ecosystems. In the case of conventional agriculture, more emphasis is laid on a linear throughput of plant nutrients (USDA, 1980; Hodges, 1981). Analysis of the theory and practice of fertilization in conventional systems (e.g. Lockhart & Wiseman, 1983), reveals that little or no attempt is made to stimulate the biological processes and systems which are the basis of the cyclical flows of nutrients observed in natural ecosystems (Cox, 1984). Conventional agricultural techniques depend on inputs of nutrients in a readily available form, given in large enough quantities to ensure that even if a substantial proportion is lost from the system (e.g. by leaching and volatilization), the remainder that is taken up is sufficient to ensure that yields will not be limited by nutrient supply (e.g. Cooke, 1977b; Cooke, 1979). In contrast, much emphasis is laid on cyclical flows of nutrients when designing organic agricultural systems (Hodges, 1981; Friend, 1983), inputs of nutrients from outside the system being used only to offset unavoidable losses of nutrients (e.g. exported in crops and lost by leaching and volatilization). A combination of careful crop and animal waste management practices and the use of agricultural practices which tend to enhance biological processes, in particular nutrient cycling mechanisms, rather than substitute for them, leads to a minimization of nutrient losses (USDA, 1980).
However, no system of agriculture (nor for that matter any natural ecosystem) can ever achieve 100% recycling efficiency. Nutrients are exported in crops to the human population from which they emerge again in a waste form, by far the majority of the nutrients incorporated therein being dumped in freshwater and marine ecosystems. At present recovery of only a very small proportion of these nutrients is regarded as an economic proposition, and in the case of some important nutrients (e.g. potassium) may never be technically feasible. Thus all systems of agriculture have to rely on some form of nutrient input if yields are to be sustained in the long-term. Where conventional and organic farming systems differ is in the form and magnitude of these inputs.
The preceding section on soil fertility examined a critical part of nutrient cycles, namely the movement of nutrients from the soil to the plant. This section will examine how the various effects of fertiliser practice listed above determine the efficiency of recycling within, and the export (in crops) and losses from, conventional and organic farming systems.
The section on soil fertility demonstrated the importance of soil structure in determining the availability of nutrients to crops. Once taken up nutrients will suffer one of three fates, namely export in crops, recycling or immobilization within the agro-ecosystem or loss from the agro-ecosystem. However, until such time as nutrients are taken up, their only possible fates are loss from the system, or immobilization within it. In the case of all nutrients except nitrogen and sulphur, losses will occur by leaching, the element entering the soil solution and being lost by percolation through the soil profile (Biederbeck, 1978; Cooke, 1982). Nitrogen can be lost by leaching (of nitrates), by microbial "denitrification" processes which involve the breakdown of nitrates to nitrogen gas or nitrogen oxides, and by volatilization, a process involving the breakdown of ammonium compounds releasing ammonia gas (Cooke, 1982). Soil structure affects not only the uptake of nutrients, but also these routes of nutrient loss, though its direct contribution is probably small in practice.
Soil structure effects on the accessibility of nutrients to crop roots was examined in the section on soil fertility. Suffice to say here, that in a poorly structured soil (e.g. one with low soil organic matter levels) crop root systems tend to be poorly developed and a higher proportion of nutrients in the soil will not be close enough to a root to be taken up (Wiersum, 1962). Under these conditions a nutrient will remain where it is until leached or immobilized by reaction with other soil constituents.
Soil structure affects soil drainage and water retention capacity, both of which influence loss of nutrients. A poorly drained, waterlogged soil can accelerate denitrification in the presence of easily decomposed organic matter (Cooke, 1982). Though organic manures provide the latter they also usually have beneficial effects on soil drainage. As decomposable organic matter is also present in inorganically fertilised soils (e.g. the stubble after cropping), impeded drainage can accelerate denitrification.
Soil structure also affects nutrient cycles by virtue of soil erosion processes. Loss of nutrients in surface runoff related to soil erosion was mentioned in part I and in the section on soil fertility above. Soil erosion and runoff from excessive applications of animal waste represent the only significant pathways for phosphorus loss (as opposed to export in crops) from agro-ecosystems (Ryden et al., 1979; RCEP, 1979). The effect of these losses on the wider environment have received more attention than the effects on soil reserves of this nutrient, and are dealt with in a later section (see p.263). Significant losses of other nutrients (e.g. potassium) by erosion and surface runoff may also occur as a result of organic matter losses and washing out of the finer soil particles such as clays, which have a high CEC and will therefore be carrying a substantial proportion of the soil's nutrient cation reserves.
Many of the chemical effects of fertilization practices in conventional and organic farming systems which impact on nutrient cycles have already been considered in the relevant sections on soil ecology (pp.231-233) and soil fertility (pp. 245-249). In terms of nutrient loss from or immobilization within agroecosystems, the most important effects are those relating to changes in soil pH and the cation exchange capacity of the soil. Further consideration of chemical effects on nutrient cycling is held over to the next section on biological effects. This is deemed unavoidable in any consideration of nutrient cycling, as these cycles are so much the product of intimately related biological, chemical (and geological) processes. Any attempt to separate chemical and biological effects would be a negation of the interactive nature of the processes which underpin these cycles.
At any one time a substantial proportion of the nutrients flowing through an ecosystem, whether natural or agricultural, will be contained within the soil organic matter (Coleman et al, 1984). The release of nutrients locked up in this organic matter is dependent on the decomposition processes wrought by a range of soil organisms. Changes in the population levels of these soil organisms consequent upon agricultural practices will result in major changes in the patterns of loss, retention and flow of nutrients in the agro-ecosystem.
This section is largely devoted to a consideration of nitrogen in agroecosystems, with only a few references to phosphorous and potassium supply as affected by different fertiliser practices. This is partly because work has been devoted to understanding the flow of N (presumably because it is regarded as the nutrient which most frequently limits crop yields), but also because it is the supply of this element which is most affected by complex biological/chemical interactions.
As Power & Doran (1984) pointed out, one would expect the raising of SOM levels (and hence the increase in C:N ratios) brought about by organic farming techniques to decrease the levels of readily available N in the soil. However, as a result of the increase in microbial (and to a lesser extent soil fauna) populations, the quantity of potentially mineralizable, and therefore available, N usually increases. The actual amount of N mineralized depends on the level of microbial activity which in turn is dependent on factors such as the availability of water and oxygen, soil temperature and the accessibility of the organic matter (Power & Doran, 1984). In conventional farming systems N is supplied in a highly soluble mineral form, so soil processes have less effect on the availability of N to the crop. There is a large pool of inorganic N in the soil, immediately available for uptake by the crop (and soil organisms) or leaching and volatilization. In fact, uptake and/or loss of mineral N occur so quickly that it may be that nitrogen levels in the total soil compartment can be raised only by organic manuring. Williams (1973), working at Rothamsted, found that the soil levels of N increased when FYM was applied, but not when inorganic N fertilisers were applied.
The efficiency of crop uptake of N from organic and inorganic sources differs in both quantitative and temporal terms. While it is true to say that immediately after application uptake is substantially greater from inorganic fertilisers, a significant proportion of the N in organic manures becomes available for uptake over a much longer time-span, effects being registered on crops planted in subsequent years. Annual recoveries of N from inorganic and organic sources noted by Widdowson & Penny (1973) were 43% and 25% respectively. Widdowson et al. (1982) estimated that N recovery from FYM was one third to half that from inorganic fertilisers depending on the crop while Smith & Unwin (1983) and van Dijk & Sturm (1983) estimated annual recoveries from organic manures as 30-40% of those from inorganic fertilisers. Recovery rates will, however, vary widely depending on soil and climatic conditions, the crop grown and the organic manure used.
Such estimates of annual recoveries of N fail to take into account the releases of mineral N which occur from organic manures in later years. Pratt et al ( 1976) developed the concept of a decay series which takes into account the variability in N contents of organic manures and progressive mineralization of this content over several years. A series of numbers represents the fraction of N of the total applied or remaining, which mineralizes in each successive year. For liquid manure with a high inorganic N content such a sequence would be 0.75, 0.15, 0.10 and 0.05. A drier manure with less inorganic N and readily decomposable organic N might have a decay series of 0.45,0.10,0.05 and 0.05 (Pratt et al 1976). Using a modified form of this concept, backed up with some experimental data but relying on certain assumptions, Sluijsmans & Kolenbrander (1977) have calculated that regular applications of an organic manure can ultimately create an equilibrium state in the soil at which the annual N supply to crops is equivalent to about 75 kg of inorganic fertiliser for each 100 kg N supplied annually in organic manures. This long-term recovery rate of N from organic manures is double that of some of the annual rates quoted above, but has yet to be verified experimentally and would undoubtedly show substantial variation under differing conditions. However, it is interesting to note that Smith (1942) found in a 50-year study that when organic manure or crop rotations were used to supply N to crop plants the efficiency of N recovery was greater than when inorganic fertiliser was used.
The differences in N availability and rates of release from organic manures and inorganic fertilisers have implications for both agricultural crops and the wider environment. Rates of release from organic manures may well match patterns of crop uptake more closely than single dressings of inorganic N fertiliser (Cooke, 1977a), clearly an advantage for crops with a long growing season, and may also result in higher N concentrations in some crops (Jenkinson & Johnston, 1977), these generally being a good indicator of protein content. Warren et al (1965) found evidence that lucerne, a green manuring crop, releases N in forms which favour its accumulation in grain and also encourage maximum yields of grain and straw.
The highly soluble form of inorganic fertilisers also means that they are generally more prone to leaching from the soil profile. Ott et al (1983) measured nitrate concentrations deep in the soil profile (120 cm) below crops receiving inorganic fertilisers, FYM and composted FYM. They found that leaching of nitrate from inorganically fertilised plots was, on average, two to three times that from the organically manured plots. Smith & Unwin (1983) list a number of studies which indicate that nitrate leaching from slurry, with perhaps the highest available fraction of N of any organic manure, is generally low if applied to growing crops (i.e. usually in the spring). In fact in some cases nitrate losses from slurry were substantially lower than those from inorganic fertilisers applied at lower rates of total N content. Suzuki et al. (1977) found that nitrate leaching losses from animal wastes were less than that from inorganic fertilisers, even when N recovery by the crop was less from the animal wastes, the residues in the soil being consequently higher. Unfortunately, there are as yet no detailed comparisons of leach rates from organic and conventional farming systems, most data so far collected relating to simple comparisons of leaching from a single organic manure and an inorganic fertiliser, both applied in the context of a conventional farming system. Koepf (1976) claims lower leaching rates for systems of organic management. The experimental results on which this conclusion is based, while showing consistently lower leach rates from organically managed plots, do not keep many of the other variables affecting leach rates constant (e.g. different crops are grown in the same year on organically and conventionally managed plots) and lack an adequate control. Power & Doran (1984) conclude in their review of N use in organic farming that these techniques tend to conserve N in the soil-plant system, often resulting in a build-up of soil organic N and that the presence of higher levels of organic residues in these systems aids in reducing N losses. The implications for the wider environment and efficient resource utilization of any differences in leach rates of N from conventional and organic farming systems are discussed in later sections.
The differences in P and K availability, uptake and losses in conventional and organic farming systems are likely to be less pronounced than is the case with N cycling. There is a considerable amount of evidence that there is little difference between the efficiency of uptake of P and K from inorganic fertilisers and organic manures. Widdowson et al. (1982) found that P recovery from FYM was similar to that from inorganic fertilisers on an annual basis, and that K was taken up in similar quantities from the two sources over a period of five years. In slight contrast, van Dijk & Sturm (1983) concluded that the P availability in animal manures and slurries was equal to that in inorganic fertilisers in the long term, while the K content of animal manures was almost exclusively in soluble form and was therefore as rapidly available as that in inorganic fertilizers.
Fraser (1984) suggested that the differences in the effects he recorded of inorganic fertilisers and organic manures on microbial populations could result in a higher potential for nutrient cycling in the organically amended soil. Hayman (1974) reviewed the importance of micro-organisms in the solubilisation and mineralization of unavailable phosphate in the soil, which results in the production of orthophosphate ions, the form of P most commonly taken up by plants. Yet micro-organisms can also effectively immobilise phosphate by virtue of the fact that they compete with plant roots for it when the supply is limited. Until such time as investigations have been undertaken into whether or not fertiliser practices interfere with the balance between microbial solubilization/mineralization and immobilization processes, the comparative effects of inorganic fertilization and organic manuring are a matter for conjecture.
The highly soluble nature of most K compounds combined with the tendency to bind very strongly with certain soil constituents (e.g. some clays, Cooke, 1967) means that availability, uptake and leaching of this element is far less dependent on biological factors than most.
There is little that can be said about biological effects on the cycling of the remaining plant nutrients. The biological components of the soil environment known to affect these nutrients have already been mentioned in the section on soil fertility. The most important effects of differences in fertiliser practice would again appear to relate directly to changes in SOM levels. High SOM levels will favour the retention of bases such as calcium and magnesium (e.g. Williams, 1973; Patriquin, 1984) and are important in the uptake of many of the micronutrients (see Allison, 1973). SOM also has an important role to play in the cycling of sulphur, being one of the three major factors (the other two being pH and precipitation) affecting the amounts of total S in agricultural soils. More than 90% of the S in most non-calcareous soils is present in organic form, and in such soils it has been shown that losses of SOM are paralleled by (proportionally smaller) losses of S (Cowling & Jones, 1970). Sulphur levels (as well as total N reserves) can be conserved by some of the measures which conserve SOM levels (e.g. the inclusion of leys in rotation with arable crops and crop residue incorporation, see review by Biederbeck, 1978).
The radically different design of conventional and organic agricultural systems may be regarded as a biological effect, as the design is determined by the methods of management of the economically productive plants and animals present in the agro-ecosystem. These organisms are clearly as much a biological component of the agro-ecosystem as are earthworms or soil bacteria.
In the context of systems design, the presence or absence of crop rotations in which sequences of arable crops are alternated with periods during which the land is down to grass, has the most profound influence on nutrient cycles. In conventional agricultural systems the trend towards specialization has led to a shift away from mixed farming and an abandonment of rotational practices (particularly in England, but less so in other parts of the UK). This need not significantly inhibit the cycling of nutrients on all livestock farms as efficient recovery and handling of animal wastes can ensure that a very high proportion of the nutrients passing through the stock are returned to the soil. Cooke (1982) noted that on an all grass farm in Switzerland using the Gulle system of collecting and handling animal manures, 80% of the N and P and 90% of the K taken up by the animals was returned to the land. In contrast, on a purely arable farm the opportunities for recycling nutrients are limited to the return of crop residues. Exports of nutrients in arable crops also tend to be much higher than those in livestock produce, and many conventional arable farms increase these losses by exporting crop residues (such as straw) in addition to the primary crop. Thus a farmer selling a crop of 6 t/ha of grain and the accompanying straw exports 120kg N/ha, 25 kg P/ha and 80 kg K/ha. Grazing livestock will, on the other hand, return virtually all the P and K they consume to the soil in excrete, as well as a substantial proportion of the N. Dean et al. (1975) estimated that cattle grazing a short grass prairie returned 63-67% of the N they removed in their urine, the remainder being lost by volatilization and in the export of animal tissues, an amount which the authors concluded did not adversely affect the functioning of the ecosystem. However, if the grass is cut and removed, nutrient exports can be greater than those in arable crops--a 12t/ha cut grass crop removes around 300 kg N. 60 kg P and 300 kg K (Cooke, 1982).
The potential for nutrient recycling on mixed, arable and livestock farms is also influenced by the fact that animal wastes are a more convenient and effective medium than crop residues for the return of nutrients to the land. In the UK, animal wastes are the medium for recycling most of the nutrients taken up by crops. Cooke (1977b) estimated that the excrete of livestock generated in 1973 contained some 840,000 tons of N. 198,000 tons of P and 690,000 tons of K, of which 500,000, 120,000 and 450,000 tons of these respective totals are returned directly to the land by grazing animals. The remainder is more or less efficiently recycled depending on the emphasis placed on waste collection and management by individual farmers. By comparison, cereal straw, the most important crop residue in terms of nutrient cycling potential contained 50,000 tons of N. 10,000 tons of P and 100,000 tons of K in 1973 (Cooke, 1977b). Half was returned to the soil as a constituent of FYM, delivering virtually all of its nutrient content to the soil. The remainder was burnt, which results in a rapid return of P and K to the soil, but loss of much of the N (and S) to the atmosphere. Animal wastes can be applied to the soil in raw form as the nutrient content is readily available to the crop whereas crop residues generally require some form of breakdown (by rotting, composting or burning) to allow the rapid uptake of nutrients needed to match a growing crop's requirement.
In more general terms, the imaginative use of livestock can be an important tool in a flexible approach to fertility management within the farm boundaries. The quality of rough pastures in upland areas where the levels of available soil nutrients are low, can be improved by grazing. Floate (1970) showed that levels of mineral N and P in upland and hill pasture soils could be raised by the decomposition of excrete from grazing sheep (the percentage of P present in potentially available inorganic form in sheep dung averaged 78.4%, compared to 62.3% in plant material). Animals can also be used to rapidly shift nutrients in the soil from one part of the farm to another. This was a critical factor in the early development of agricultural systems, allowing a build up in the fertility of land used for arable crops by applications of FYM generated by fodder crops grown on other land (Larsen, 1976). Such practices can still have a role to play in fertility management on present-day farms.
In addition to the nutrient cycling advantages offered by animal manures, there are also important gains in soil fertility accruing from grass leys, especially those with a leguminous component (e.g. clover, trefoil, lucerne etc.). The beneficial effects on SOM levels and consequently on soil structure and crop yields have been discussed above, the increase in the N status of the soil has not. Clement & Williams (1967) found that a grazed, unfertilized 3 year ley of ryegrass caused an increase in the N status of the soil equivalent to that achieved by three applications of 110 kg N/ha/y. Clement (1961) was able the ley included white clover, the improvement in N status was equivalent to that achieved by three applications of 110 kgN/ha/y. Clement(1961) was able to show the increase in N status of soils under leys was the critical factor influencing the increase in arable crop yields in subsequent years. In contrast, soils that have grown non-leguminous arable crops for many years contain the least reserves of organic N and mineralization produces the least nitrate (Cooke, 1982). This can result in a restricted uptake of N by crops in all-arable rotations (Clement, 1961) and lower yields.
The continuous arable cropping that has become so much a feature of modern conventional farming systems can result in depletion of micronutrient reserves. Conventional fertiliser practice concentrates on replacing the macronutrients removed, particularly N. P and K, but also calcium and magnesium on many farms (Lockhart & Wiseman 1983). In such systems, particularly those adopting monocropping practices, where organic manures which contain the full complement of plant nutrients are not being applied, micronutrient deficiencies are likely to become a problem (Cooke, 1982). It may well be that the importance of organic manures in recycling these nutrients and minimising their loss from agro-ecosystems has been underestimated. Unfortunately, the opportunities for reaping this and the other listed benefits of a return to more efficient nutrient recycling in UK agriculture, have been severely limited by a single major effect of the continuing trend towards specialization in conventional farms. Most of the livestock production is now concentrated in the North and West of the country, whereas most of the arable production is confined to the more climatically favourable eastern half of the country. Transfer of bulky organic wastes (whether animal or plant) with a low nutrient content across the country is simply not feasible (see Vine & Bateman, 1981).
Systems design may well be the most significant biological effect on nutrient cycling (Atkinson, 1986). The major detrimental effect of inorganic fertilisers could be that they have made it possible to undertake specialized farming (without rotations) for extended periods in many areas (Koepf, 1986). Apart from considerations of the consequent soil degradation (sustainability is to a large extent a function of soil:crop interaction), this type of farming is based on the simple doctrine of maximising the yield of each individual field, an objective which can only be achieved at the cost of substantial nutrient losses from the system. Organic/biological and biodynamic farming systems are designed as an interacting set of enterprises in a site-adapted programme, the careful programming and assembly of these enterprises serving to minimise the nutrient inputs necessary to maintain output.
This section is primarily concerned with effects of inadvertent loss of nutrients from agro-ecosystems to neighbouring natural and semi-natural environments. The most significant losses in qualitative and quantitative terms are those which occur in water draining from agricultural land, and to a lesser extent in surface runoff of water and eroded soil. The gaseous losses of N from agro-ecosystems are also discussed, but the impact of these losses on the global environment remains unclear and may not be important. Changes in invertebrate soil fauna wrought by fertiliser practices may have some effect on the natural vertebrate fauna present in agro-ecosystems and associated natural and semi-natural habitats. The possible nature of these effects as well as documented effects on floral species diversity in agro-ecosystems are to be reviewed in part IV of the report which considers farming systems impacts on wildlife and its habitats.
In terms of environmental quality, N and P are far and away the most important nutrients leached from agricultural land. The enrichment of surface water bodies (streams, rivers and lakes) and groundwater (all subsurface waters, and in particular the reserves in aquifers) has serious implications for the flora and fauna that inhabit surface waters, and the human populations that draw upon both surface and groundwater supplies. Surface and groundwater enrichment will be dealt with separately here, as the mechanisms controlling nutrient import to these systems are different, the relative importance of N and P in the two processes differs widely, and the brunt of the environmental effects fall on different elements of natural and human communities.
Eutrophication may be defined as any process which adds to natural waters substances that are nutrients for micro-organisms and plants, or which stimulate their growth (Cooke, 1982). The nutrients chiefly involved in this process are nitrates and phosphates, which are frequently limiting in nature, though other less important nutrients (e.g. magnesium, potassium, sulphates and trace elements) may have some effect too (Maitland, 1984). In most freshwaters N is much more abundant than P. so that it is the latter which is usually the critical factor limiting natural production. It is often the case that only very small increases in P levels in surface waters are needed to stimulate the algal blooms which initiate qualitative and quantitative changes associated with eutrophication in aquatic communities (Holt et al ,1970).
The human activities which contribute to nitrate and phosphate enrichment of surface waters are industry, agriculture and the disposal of human wastes. The most important source of nitrates is generally agriculture, between 10-25% of N applied as fertiliser entering adjacent running waters (Maitland, 1984). Holden (1976) estimated that some 85% of the N input to Loch Leven was from agricultural land and that this represented perhaps a third of the fertiliser applied in the catchment area of the loch. In contrast, the same loch received only 15% of its P input from agricultural sources, most of the remainder coming from industry, though sewage was also an important source (Holden, 1976; Holden & Caines, 1974). In his review, Maitland (1984) indicated that domestic sewage was generally the most important source of P in surface waters. Thus while P input to surface waters almost invariably has the greatest impact on the eutrophication process, it would appear that agriculture is generally not the most important source of this P. Furthermore, the tendency of P compounds to bind strongly to soil constituents and remain in insoluble inorganic and organic forms means that whereas nitrate entering surface waters from agricultural land does so in solution, P usually enters bound to particulate matter or in insoluble form. These insoluble P compounds are often biologically unavailable so that their potential impact on aquatic ecosystems is further reduced (Ryden et al.,1973). Nevertheless, it is still worth attempting to compare the effects of conventional and organic farming systems on P input to surface waters, as the impact of these inputs may, in some cases, be important (e.g. in rural areas with a low human population density and an absence of industrial development).
In as much as soil erosion is far less of a problem in organic than in conventional farming systems (USDA, 1980; Reganold et al., 1987; Arden-Clarke & Hodges, 1987), phosphorous inputs to surface waters will be lower from organically farmed land. Cooke (1976) states that, in Europe at least, the main agricultural contribution of phosphates to the eutrophication process is consequent upon soil erosion. Ryden el al. (1973) noted that in the US the highest concentrations of P measured in surface runoff were from land under "row" (arable) crops. This presumably relates to the greater susceptibility of arable land to soil erosion, and obviously has serious implications for the continuous arable cropping practices that have become characteristic of so many conventional farming systems.
Intensification of livestock husbandry practices can also increase P inputs to neighbouring water bodies--Ryden et al. (1973) found that intensive animal husbandry units can constitute important point sources of P. This is also a problem in the UK, which can be exacerbated by over-stocking of pastures with grazing animals and injudicious spreading of animal manures and slurries (Cooke, 1976). This raises the general point that organic manures may not be, intrinsically, any less of a pollution problem in terms of P leaching and runoff, than inorganic fertilizers. The critical difference in fertiliser practice between these contrasting farming methods is to be found in the respective approaches to the collection and handling of the animal wastes generated by livestock farming. Intensive livestock husbandry units have no place in organic farming systems, stocking rates tend to be on average about 70% of those in conventional systems (Vine & Bateman, 1981) and animal manures are carefully conserved and utilised resources which are almost invariably in short supply on organic farms.
Some organic farmers do apply mineral phosphate fertilizers, but they use rock phosphate and basic slag which are far less soluble than the synthetically compounded fertilisers such as superphosphate used by conventional farmers. This difference may not be critical in view of the strong binding of phosphates to soil constituents, but it has been noted that residues from superphosphate are less effective in promoting crop yields in later years than are less soluble P sources (e.g. Roy & Thomas, 1951; Roscoe, 1960) . Therefore more frequent dressings of superphosphate (and consequently greater quantities of P) are needed to maintain yields, a practice which may be reflected in increased P losses from the agro-ecosystem. However, the rise in P fertilization has accompanied the intensification of conventional farming methods has not been as dramatic as the rise in N fertilization (Green, 1973), and tailed off some years ago.
Whatever the quantitative differences in P inputs from conventional and organic farming systems, the qualitative effects on aquatic ecosystems are not in any doubt. The effects of eutrophication can be summarised (e.g. Maitland, 1984) as: rapid increases in phytoplankton species (especially blue-green algae in some water bodies); enhanced growth of some macrophytes which later die back; a reduction in invertebrate species diversity; and marked changes in the fish fauna (e.g. salmonids being replaced by coarse fish). Primary production may be raised so high that subsequent decomposition of dead plant matter and plant respiration at night may completely deoxygenate the water, eliminating all fish. The costs to human society of eutrophication are difficult to evaluate, but are undoubtedly high in economic (e.g. on salmon fisheries), environmental (water quality in general) and recreational (e.g. on angling and boating) terms.
The affinity of dissolved inorganic P for certain soil minerals ensures that little P reaches the groundwater reserves after percolation of soil water through the soil profile. Only in rare cases where the proportion of P-retaining compounds in the soil profile is low will significant quantities of P turn up in subsurface and groundwaters (Ryden et al., 1973). This part of the account is restricted to consideration of nitrate enrichment (also termed contamination in view of the possible risks to human health) of groundwaters. It should be noted that some other nutrients leached from agricultural soils do reach groundwater reserves, at present only in small quantities. Foster et al., (1982) noted that there has been an increase in the leaching and/or mobility of calcium, sodium, magnesium, potassium, barium, strontium, boron and possibly other ions, concomitant with the recent increase in nitrate leaching rates. These may be a direct result of modern farming practices or an indirect result of the modification of water-rock equilibria in the unsaturated zone of aquifers, caused by the changing composition of soil leachates. Though these other ions are ultimately likely to cause further water purity problems (Foster et al., 1982), little else can usefully be said about them on the basis of present knowledge.
Regardless of the original fertiliser source, most N compounds applied to the soil are transformed to nitrate which is not strongly held by any soil constituent. In a well drained soil all this nitrate, which is not taken up by plants and microbes or denitrified by denitrifying bacteria, will ultimately be leached from the topsoil and subsoil (Cooke, 1967). The leached nitrate will steadily accumulate in the unsaturated and saturated zones of aquifers underlying N fertilised agricultural land. Groundwater from aquifers which underly extensive tracts of agricultural land, provides some 30% of developed supplies of potable water in Britain (Foster et al., 1982). The potential of nitrates in drinking water to pose a health risk to exposed human populations has yet to be accurately quantified, either in respect of carcinogenic effects on the population as a whole or the potentially fatal condition of methaemoglobinaemia in infants. However, limits for "recommended" (< 11.3 mg NO3N/1), acceptable (11.3-22.6 mg NO3-N/I) and unacceptable (> 22.6 mg NO3-N/I) levels in the water supply have been set by the World Health Organisation (WHO). The recommended and acceptable limits have already been exceeded in the unsaturated zones of some aquifers below long-standing arable land in the eastern half of the UK (Foster et al., 1982). The widespread ploughing of grassland, principally during the early 1940's, provided the initial impetus for the rise in groundwater nitrate levels (Young 1986). The intensification of arable practices since that time, with the accompanying increase in use of inorganic nitrogen fertilisers, has reinforced and extended this trend. Future impacts on groundwater quality are also likely to depend on changes in the pattern of fertiliser usage. White (1983) predicts a rise in nitrate concentrations in groundwaters under grassland catchments as a consequence of the projected increases in fertiliser applications to grassland. This author suggests that the soil nitrate available to water is likely to continue to increase for the forseeable future. Foster et al., (1982) suggest that if the present trend continues there may well be a need for alternative water supplies in some areas from the 1990's onwards.
A wide range of factors influences the rates at which nitrates are leached from agricultural soils (e.g. the nature of the soil, climate, cropping systems), of which fertiliser practice is only one. As yet there have been no studies completed which adequately compare nitrate leaching rates in conventional and organic farming systems. This problem was first touched upon in the section on biological effects relating to nutrient cycles, where the issue of comparisons of nitrate leaching rates from inorganic fertilisers and organic manures was examined. Most such comparisons were of individual organic manures and inorganic fertilisers, made in the context of conventional farming systems. Evaluating the impact of conventional and organic farming systems on groundwater nitrate levels is fraught with even more problems. Given the slow rate of nitrate leaching through the soil profile (estimated at I m/y in southern England; Cameron & Wild, 1984) and the mixing of nitrates from various sources (e.g. inorganic fertilisers, organic manures, mineralized soil organic matter) it is impossible to measure directly the impact of any one nitrate source on groundwater quality. It is therefore only possible to look at some of the broader aspects of agricultural systems design and evaluate how they might affect nitrate losses to groundwater. Fertiliser practice is one relevant aspect of systems design which will be dealt with first.
Such direct comparisons of nitrate leaching through soil profiles from inorganic fertilisers and organic manures so far undertaken, have concentrated on animal slurries. This is presumably because the generation of large volumes of these liquid manures by intensive animal husbandry units has created major disposal problems. Vetter & Steffens (1981) note that, in general, it has been found that leaching of N after the application of slurry is higher than after the application of inorganic N fertilizers. This was because: (i) a higher proportion of slurry is spread in the autumn; and (ii) the rates of slurry N applied are generally higher than the rates of fertiliser N applied/ha. These poor waste management practices are a characteristic of some conventional farming systems and not of organic systems (see section on biological effects relating to nutrient cycles, p. 255). Furthermore, organic farming systems do not give rise to liquid manures which contain nitrogen in highly soluble forms (unless they constitute an important component of fertility management procedures). Solid wastes such as farmyard manure (FYM), a mixture of animal wastes and straw, are the norm in these systems, and they are stored and spread in such a way as to maximise nutrient transfer to the crop. Where slurries are used they are normally aerated to stabilise their nutrient content.
Nitrate leaching rates consequent upon organic manuring practices other than slurry (and to a lesser extent, FYM) application have so far received little attention. Green manures and other leguminous crops often represent the main source of N in organic farming systems, animal wastes acting only as a N supplement, their primary function being to recycle P. K and other nutrients. Grass/clover swards in the UK have been shown to fix between 74-280 kg N/ha/y in field experiments (Cowling, 1982). These are maxima and minima, however, most such swards fixing between 150-200 kg N/ha/y--in any case, such N inputs, even from a biological source, are likely to increase nitrate leaching rates. Wild & Cameron, (1980) reported some data showing significant leach rates from growing clover crops (30 kg N/ha/y) and marked increases on death or cutting of the crop (131 kg N/ha/y). However, in organic systems clover is rarely used as a pure crop, but is undersown in arable crops or as a mixed sward with grass. Such combinations are likely to result in much lower leaching rates as the constant low rate of N-fixation by the legume will be matched by prompt uptake by the accompanying main crop (Dobereiner, 1977). This is to some extent borne out by the findings of Garwood & Ryden (1986), who showed that nitrate leaching rates from inorganically fertilised grass swards were substantially lower than those from unfertilized grass/clover swards (which are sown in organic farming systems). When the swards were cut, nitrate leaching rates from the inorganically fertilised grassland were nearly 15 times those from the grass/clover mix. When they were grazed, leach rates from the inorganically fertilised sward were still seven times higher than those from the grass/clover fey. The undersowing of main crops with clover and other legumes and the use of grass/clover mixes as opposed to pure grass mixes, have been suggested as substitutes for inorganic nitrogen fertilization, which would result in a reduction of nitrate losses (Phillips 1986). It is, however, not necessarily safe to assume that any main crop will have nitrogen uptake rates that match those of an undersown legume--many cereals which absorb the majority of their N requirement in the space of a few weeks (about four) are a case in point (Atkinson, 1986).
The maintenance of crop cover and the planting of catch crops or green manures to avoid leaving the ground bare at times of high rainfall, have also been suggested as measures to reduce nitrate leaching (Henin, 1986). These practices are characteristic of organic farming systems but have been largely abandoned in conventional systems (e.g. AAC, 1970). The incorporation of crop residues, and in particular straw after cereal harvests, has also been suggested as a means of reducing nitrate losses (Henin, 1986; Phillips, 1986). Whereas many conventional farmers continue to burn or otherwise remove much of the crop residue from their fields, crop residue incorporation is a common practice among organic farmers (Vine & Bateman, 1981). Conversely, the organic farming practice of using short-term leys (usually mixes of grass and clover or some other legume) may have a further impact on groundwater quality. The autumn ploughing of temporary grassland on a chalk aquifer in southern England has been shown to result in the leaching of approximately 100kg N/ha over the course of two years (Cameron & Wild, 1984), resulting in NO3-N concentrations in the soil profile in excess of WHO limits. The overall effect of organic management practices is unknown as this experiment was carried out in the context of a conventional agricultural system. At present, the only figures available for nitrate concentrations in the soil profile below an organic ley-arable rotation are those of Young & Gray (1978). These indicate nitrate concentrations rising no higher than the lower end of the "acceptable" range (11.2-22.6 mg NO-71) recommended by the WHO. However, only one complete profile was monitored, from one field, on one organic farm.
Leaching of nitrates from agricultural soils remains a complex issue which deserves much further attention. Until fairly recently, it was widely assumed that leach rates were greater from arable land than pasture. However, Ryden et al. (1984) have shown that leaching rates from an intensively managed, grazed grassland, receiving 420 kg N/ha/y in inorganic form, or 450 kg N/ha/y in organic form (FYM and slurry),were substantially greater (162 kg N/ha/y and about 152 kg N/ha/y respectively) than those under arable crops (range 40-lOOkg N/ha/y). Grazing as opposed to cutting the sward had increased leaching rates by a factor of 5.6. Again, this experiment was carried out in the context of a heavily stocked conventional farming system and it is by no means clear that nitrate leaching problems attributable to the grazing of organically managed swards(which in any case would not receive such large N inputs) would be as serious. However, grazed short-term leys are a very important component of most organic rotations used in the UK and this phenomenon serves to emphasise the need for the development of leaching studies in fully established organic farming systems.
Nitrogen and sulphur are the only plant nutrients that can be lost from the soil in significant quantities by volatilization. Biological denitrification in the soil produces nitrogen gas (N2) and nitrous oxide (N2O) from nitrates, these gases subsequently entering the atmosphere. Breakdown of ammonium salts generates gaseous ammonia which is also lost by volatilization. Nitrous oxide, under the influence of solar radiation, can give rise to products (e.g. nitric oxide, NO) which catalyze the destruction of the stratospheric ozone layer (RCEP, 1979). The ozone layer filters out a substantial proportion of the ultra violet wavelengths of solar radiation, reducing the exposure of all living organisms to this carcinogenic influence. Anthropogenic sources of nitrogen oxides are also implicated in recent reductions in the pH of natural precipitation, a major cause for concern in view of its widespread and often dramatically detrimental consequences (e.g. NCC, 1984).
The problem is that there remain so many uncertainties over the mechanisms involved in both these environmental perturbations, and more specifically in the importance of the agricultural contribution to them, that no firm conclusions can be drawn on the magnitude of the threat or the nature of appropriate remedial action. It was noted, for example, in the Seventh Report of the Royal Commission on Environmental Pollution (RCEP), that there was evidence for nitrous oxide having suppressive as well as catalytic effects on reactions in the stratosphere which destroy ozone (RCEP, 1979). The report concluded that there were so many uncertainties and so much still to be learnt that "no immediate corrective action" was required. Notwithstanding all these uncertainties, it may yet be worth attempting to compare the potential of conventional and organic farming systems for release of nitrous oxide to the atmosphere.
The only direct comparisons of the evolution of nitrous oxide from inorganic fertilisers and organic manures have involved animal slurries. The large readily decomposable fraction and high mineral N content of these organic manures (e.g. van Dijk & Sturm, 1983) result in their being far more prone than others to N loss by leaching and volatilization. Indeed they have proved to be more prone to denitrification than inorganic fertilizers, Christensen (1983) showing that nitrous oxide losses from grassland fertilised with slurry were up to eight times higher than those from grassland fertilised with ammonium nitrate. Burford et al. (1976) recorded similar results for pasture fertilised with large applications of slurry and sodium nitrate. Even if these slurries were not used as a nutrient source for subsequent crops, they would still exist as an inevitable by-product of animal husbandry and would presumably still contribute to atmospheric levels of nitrous oxide. It is inorganic fertilisers which represent an additional source of these nitrogen oxides. Indeed, McElroy et al. (1977) suggested enhanced re-cycling of animal wastes could reduce the requirement for inorganic fertilizers, thus reducing the anthropogenic contribution to atmospheric nitrous oxide levels. This is precisely the effect that a shift to organic methods of agriculture would have.
The inherent fertility of soils and the non-renewable reserves of some plant macronutrients and, to a lesser extent, fossil fuels are the resources most affected by agricultural fertility management techniques. The fertility of the soil is itself a resource, which may be squandered or safeguarded according to the agricultural techniques applied. It is a resource that has yet to be quantified in anything except the crudest terms (e.g. depth of soil available for rooting, reserves of certain nutrients in the soil). Yet examination of the comparative effects of conventional and organic farming practices, detailed above and in part I of this report should satisfy an objective reader that conventional agriculture poses a far greater threat to soil fertility. Increasing problems with soil erosion in the UK (e.g. Arden-Clarke & Hodges, 1987; Evans & Cook, 1986), and the huge Federal land retirement programmes currently in operation in the US, are eloquent testimony to the fact that some agricultural soils are giving way or have already given way under the physical, chemical and biological pressures imposed by conventional agriculture. In this situation it is highly pertinent to question the need for detailed quantification of the degradative effects before remedial action is taken to halt these alarming trends. Estimates of nutrient losses due to soil erosion alone on US cropland suggest that as much as 50 million tonnes of N. P and K are lost annually (Wadleigh, 1968 cited in Pimentel et al., 1976). Willis & Evans (1977) calculated that the annual cost of replacing nutrients lost in soil eroding at a rate of 11.2 t/ha/y (the maximum "tolerable" rate) as $59/ha. If all the 146 million ha of US cropland was subject to this rate of erosion, the annual cost to the nation in terms of nutrient loss alone would be $8.6 billion. More detailed assessments of degradative effects on all the soil constituents and processes which contribute to soil fertility are simply not available and may never be quantifiable.
Soil erosion represents a gross and relatively easily detectable form of soil degradation. However, there are other forms of degradation, which, while being more difficult to detect or taking longer to become apparent, may also have important implications for the maintenance of soil fertility. A case in point is the overall reduction in soil organic matter levels which occurs under intensive, conventional arable cropping systems. Long-term experiments carried out at Rothamsted and Woburn (in some cases for periods of more than one hundred years) indicated a general lack of yield benefit from extra organic matter until the late 1970's (Johnston, 1982; 1986). Results obtained up to that time indicated that yield losses consequent upon reductions in SOM levels could be made up by applications of inorganic fertilizers. More recently, it has become clear that larger yields are being obtained on soils with extra organic matter, at both Rothamsted and Woburn. Johnston (1986) ascribes these larger yields to some combination of extra water-holding capacity, the availability of N in ways that cannot be mimicked by dressings of inorganic fertiliser, and improved physical properties of the soils with higher SOM levels. These results were generally obtained where SOM levels were very low or where large yields had been obtained (Johnston, 1982). The major conclusion to be drawn form this by Johnston (1986), was that if intensive, conventional cropping regimes remain the norm in commercial practice, levels of organic matter could well decline to the low values at which benefits could be gained by raising organic matter levels.
The fundamental difference between fertility management on conventional and organic farms is in the degree of reliance on external inputs of plant nutrients and in the handling of nutrient rich wastes generated during crop and livestock production. On conventional farms much, or even all, the agricultural waste generated may be treated as just that, the only consideration determining its handling being the economic one of finding the cheapest disposal route. The nutrients lost in these wastes and exported in crops are then replaced by a range of synthetically compounded fertilizers, the majority of which are inorganic compounds in highly soluble form. The synthesis of these products requires energy, almost invariably in the form of fossil fuels drawn from the world's finite reserves. The major fossil fuel inputs to agriculture are in the fuel used for both machinery operations and the synthesis of inorganic fertiliser--Pimentel (1980) calculated that of the total input, fertiliser synthesis accounted for some 30% of this energy expenditure. In their review, Parr et al. (1983) stated that the net reduction in energy expenditure from substituting animal manures for inorganic fertiliser ranged from 15-25% when the manure source was in the vicinity of (about 5 km or less) the site of application. However, they also noted that most of the energy saved by avoiding synthesis of inorganic fertilisers is in the form of natural gas, and most of the energy used in applying manures and organic wastes is in the form of petrol or diesel fuel. Therefore, substitution of organic wastes for inorganic fertilizers, while resulting in less total fossil fuel consumption would cause an increase in the consumption of these liquid fossil fuels. Efficiency in terms of yield for a given energy input is apparently greater on organic farms as total energy inputs are lower (Lockeretz et at, 1976), but Parr et al. (1983) noted that productivity (in terms of energy content of the crop) per hectare of cropland may be lower on organic farms.
In contrast, organic farms are managed in such a way as to maximise internal cycling of nutrients, thus minimising the requirement for external inputs. There is currently much scope for improving the efficiency of collection, storage and application of animal wastes on UK farms. The total value of the nutrients contained in FYM and slurry handled (i.e. not including manure dropped directly onto swards by grazing animals) annually in the UK has been estimated as £160 million (Smith & Unwin, 1983). In 1981 the quantities of N. P and K in those animal manures represented 37%,65% and 97% respectively of these macronutrients supplied that year in inorganic fertiliser form (calculations from Smith & Unwin, 1983; Church, 1982). Similar figures are not yet available for the nutrients contained in crop wastes.
The unavoidable nutrient deficit consequent upon exports in crops and various other loss routes (e.g. by leaching and volatilization, features of any nutrient cycle) are replaced as far as possible by off-farm organic wastes. Where the supply of such wastes is limited, some macronutrients losses (particularly P and K) can be replaced in mineral form (Koepf et al., 1976; von Fragstein & Vogtmann, 1983: van Fragstein et al., 1986), usually as unrefined ores or byproducts of industrial processes, for example rock phosphate, basic slag (for P) and glauconite (for K). The unrefined nature of these mineral fertilisers minimises their cost in terms of the energy consumed during their production. Their relatively insoluble form is also likely to reduce the losses attributable to leaching processes.
Unfortunately, there are as yet no detailed comparative assessments of nutrient flows and budgets on conventional and organic farms. Preliminary data gathered on a Biodynamic farm in the Netherlands (Vereijken, 1986), indicate that crop requirements for N can be met from a variety of organic sources (e.g. biological N fixation, animal manures, off-farm organic wastes), but that there is a P and K deficit which would presumably have to be replaced by an off-farm source (possibly in mineral form) if chemical weathering of the soil and bedrock is not sufficient to rectify this loss. In conventional agricultural systems the problems associated with nutrient budgets tend to be ones of oversupply rather than deficiency. Cooke (1977b) estimated that the amount of N involved in soil-crop-livestock cycles in the UK was about twice as much as that taken up by crops and grass grown annually. This implies that there are large N losses from UK agricultural systems. Cooke also noted that more P than can be utilised by crops was applied to UK soils, in the form of both inorganic fertilisers and animal wastes. The surplus tends to accumulate in the soil due to its strong binding properties so most is not lost from the system. Nevertheless, Cooke estimated the amount of P applied annually could be reduced by 30-50% without affecting crop yields. In the long term such savings could prove important as though there is no immediate prospect of exhaustion of the world's reserves of P. it has been calculated that at the present price, reserves of this macronutrients will be the first to run out (Frissel, 1978). There are, however, reserves in existence that would become economically mineable if the price increases. There is apparently less scope for reducing inputs of K, but some economies could be made by improved storage, handling and application of animal wastes and reducing K dressings on soils in which clay minerals release much of this element.
The disposal of human excrete in the form of sewage sludge and effluent (usually to aquatic ecosystems) represents a major nutrient loss from any agricultural system. The adoption of organic farming techniques is obviously not, in itself, a solution to this problem, which constitutes a major barrier to the development of truly resource efficient agriculture. In fact the potential for the adoption of organic methods might ultimately be limited if no attempt is made to close this part of the nutrient cycle. At present the contribution of treated human waste (in the form of sewage sludge) to the nutrient requirements of crops grown in the UK is minimal. Smith & Unwin (1983) estimated that the fertiliser value of the total annual UK production of sewage sludge represented only about 2.5% of the N, 5% of the phosphate and 0.5% of the K applied annually in organic form. Furthermore, only about half this sludge is actually applied to the land.
The major factor limiting the return of these nutrients to agricultural soils is the inefficiency of the sewage treatment process which results in much of the N and P and virtually all of the K being discharged in the liquid effluent (Cooke, 1977b). Yet sewage treatments have been devised that would increase the proportion of nutrients recovered and reduce the pollution of the wider environment. Of the macronutrients mentioned above, prospects for an improvement in the recovery rate of P are best, and also have important environmental implications. At present some 50-60~o of P that passes through sewage treatment plants (a substantial proportion of which originates from domestically used detergents) is discharged in the liquid effluent, which in turn contributes over 90~O of the total P loading in rivers in England and Wales (Williams & Coker, 1981). Addition of iron or aluminium salts or calcium hydroxide can remove 66-98% of the P contained in the untreated sewage (Bayley, 1970). Lassen et at (1984) have estimated that extended waste water treatment to enhance recovery of P could result in sewage sludge P substituting for 30-100% of all imported raw phosphates used in Danish agriculture. As the costs of importing P increase this option may well become economically viable using existing technology. The potential for P recovery may not be as high in other countries and certainly does not currently approach anything like this recovery rate in any country.
The prospects for N and K recovery from sewage do not match those for P. Much of the N in sewage sludge occurs as highly soluble nitrate and virtually all the K occurs in soluble, inorganic forms, making extraction prohibitively expensive or even technically impossible. Where N occurs in forms that can be recovered (e.g. ammonium compounds) there remains the problem of limiting volatilization and denitrification losses after recovery. A partial solution to this problem might be provided by blending and composting sewage or sewage sludge with bulking materials such as woodchips or other solid organic wastes (Witter, 1986). The USDA has been able to produce a marketable fertiliser/soil conditioner in this way, which takes the form of a humus like material which is both odour and pathogen-free (USDA, 1980).
Recycling of both agricultural livestock and human wastes is not without problems. With respect to livestock wastes some of these problems have already been mentioned above (e.g. see section on wider environmental effects of fertiliser practice). Many of these problems are directly attributable to the large surpluses produced by intensive animal husbandry units, which are not a feature of organic farming systems. One particular problem not mentioned above was that of animal manures with potentially toxic feed additives, in particular certain heavy metals. Zinc is often added to poultry feedstuffs and copper to pig feed to promote the growth of these animals in intensive units. Repeated applications of manures originating from such units could therefore lead to a build up of these elements to toxic levels in the soil. Feed additives such as these are not used by organic farmers, but where they are importing animal manures from conventional farms, or indeed where conventional farmers are applying these wastes, the heavy metals could present a long-term problem. However, in the case of copper in pig manures at least, Dight (1983) has noted that future trends even in conventional agricultural systems are towards a more balanced mix of livestock and arable enterprises to allow application of slurry at rates insufficient to cause a dangerous build up of copper. This problem could in any case be avoided by the simple expedient of discontinuing (or at least minimising) the use of these additives.
Other problems often associated with the application of animal wastes to agricultural land include the survival of animal pathogens, the generation of offensive odours and the impairment of pasture palatability (e.g. McAllister, 1974 & RCEP, 1979). The first two problems can be avoided by composting of the wastes (the second of these also by simple aeration), the latter by careful timing of applications (e.g. McAllister, 1974). Solution of such problems can usually be achieved by thoughtful design of the waste management systems on a farm, such design being a major feature of organic farms.
The problem of heavy metal contaminants in sewage sludge is likely to prove rather more intractable. Voorburg (1983) noted that only 42% of sludges used in Dutch agriculture in 1981 met that government's guidelines of maximum allowable levels of certain heavy metals (these guidelines are generally more restrictive than those of the EEC). Cadmium is a particular problem as uptake by the crop seems to be easier than for other heavy metals, and human daily intakes, mainly with food, are increasing (Voorburg, 1983). Even if a farmer applies sewage sludge with a cadmium content below the maximum level recommended by the EEC, there will still be a build up in soil levels of this element. The potentially detrimental effects of heavy-metal contaminated sewage sludge on the biological activity of soils, and hence on the fertility of organic systems, has been recently demonstrated (Long, 1985; McGrath et at, 1987). This work showed that, in a soil treated with sewage sludge between 1942 and 1961, even after 25 years the residual effects reduced the microbial biomass to half the control level, and considerably reduced the symbiotic and non-symbiotic nitrogen fixation. Much more research is needed on the problem of heavy metal contamination of sewage sludge before any firm conclusions can be reached on the dimensions of the problem or the measures necessary (and feasible) to prevent human exposure to toxic levels of these elements. In the long term, if sewage sludge is to be used extensively as a fertiliser, the only solution may be to separate domestic and industrial sewage systems, as the great majority of heavy metal contamination is of industrial origin. Alternatively, legislation could be introduced to prevent the relevant industries dumping toxic wastes into the sewers. The companies involved are simply externalizing a cost, which is then borne by society as a whole--application of "the polluter pays" principle is long overdue in this instance. A combination of a reduction in the industrial effluent contamination of domestic sewage and a switch to sewage treatments which enhance nutrient recovery could substantially reduce nutrient losses consequent upon human waste treatment and disposal.
As with livestock waste there is the problem of pathogen survival in sewage sludge. But this can largely be avoided by intelligent use of organic wastes (e.g. livestock waste application to arable crops grown for direct human consumption, sewage sludge application to fodder or grass crops) or specific handling techniques (e.g. composting). Dight (1983) notes that there are currently no livestock or human health problems attributable to the application of sewage sludge to grazing land in the UK.
The scope for drawing firm conclusions from the foregoing review is limited by gaps in our understanding of soil ecosystems in general and soil fertility and nutrient cycles in particular. Furthermore, the effects of conventional farming systems on the delicately balanced relationships between the physical, chemical and biological components of the soil are only gradually coming to light. Those of organic farming systems have to be predicted, on the basis of
extrapolation from the known effects of individual practices which characterize these systems, as few such systems exist and little research has been devoted to them. The most pertinent conclusion is therefore that far more research is needed on the factors controlling soil fertility, the effects of various agricultural practices on these factors and the gathering of empirical data on the comparative effects of conventional and organic systems on the soil.
Research should be conducted on the various organic manures and organic manuring techniques, used in conjunction with each other rather than in isolation, to establish recommendations for their use similar to those which exist for inorganic fertilizers. Experimentation with farm systems design should be conducted with the objective of maximising the internal cycling of nutrients and minimising losses and inputs of these elements. As an adjunct to this, more effort should be devoted to the development of efficient waste collection, handling and distribution systems. Off-farm sources of organic waste should not be ignored as these could contribute substantially to balancing unavoidable nutrient losses from farms. Where research into crop nutrition is aimed solely at maximising yields, the objectives of the programme should be broadened to include sustainability. Newbould (1982) has previously recommended that equilibrium levels of organic matter, and those at which yields, structure and erosion potential are affected should be determined or estimated from existing models for the range of soils, climates and farming systems in Europe. Deleterious effects on the soil should be minimised and the full resource costs of the output achieved, established. In this respect Newbould (1982) called for the establishment of multidisciplinary agro-ecosystem experiments, to collect data on all aspects of the processes determining crop production and transfer of nutrients to other ecosystems.
Intensive conventional agricultural systems and practice are as yet too recent innovations to have been put to the test of sustainability (Lovett, 1980; Ulbricht, 1981). The conventional approach to the provision of crop nutrients and maintenance of soil fertility has grown out of an oversimplified model of a barely understood system. The fundamental nutrient requirements for crop growth were established (or at least those which are most usually limiting under natural conditions) and these are provided in synthetic form, usually in excess of crop requirements. The overall approach is effectively one of substituting large external inputs of readily available nutrients for the natural soil processes which make available a continuous supply of these elements. This has proved very effective in the short-term, and may even prove sustainable beyond the short-term on particularly resilient soils. On other soils gradual loss of fertility may not prove to be the critical degradative process, more dramatic effects such as soil erosion preceding and masking the more subtle degradation. The form the degradation takes is to some extent irrelevant, so long as the cause is clear. In this respect Newbould (1982) notes that the halving of SOM levels of soils in the US and Canadian plains has been accompanied by a decline in the cereal production of those areas. This and other evidence cited above suggests that the accumulation of the various sideeffects of conventional fertiliser practice will ultimately lead to the collapse of some agricultural soil ecosystems and a consequent loss of soil fertility, which in some cases could prove irreversible.
It would seem that a prerequisite for confronting the issue of soil fertility, is an enhanced appreciation of the importance of natural processes in soil fertility and nutrient cycles and the effects of the substitution of these processes attempted by conventional agricultural practice. Indeed, it is valid to question the presumption implied by the efforts made to devise a substitute for processes yet to be adequately modelled. Fertiliser practice should be designed with a view to the enhancement of biological processes controlling nutrient flows in agricultural soils, not with the objective of substitution. In this respect, the advantages of organic over conventional methods are irrefutable. Furthermore, the indications are that organic fertilization practices will be more "resource efficient", especially in terms of world reserves of (recoverable) nutrient elements, fossil fuels, soil, and the wider environment. Realisation of these benefits requires a prompt redirection of agricultural research and development towards optimising the design of organic farming systems and away from reductionist approaches which seek only to maximise yields without regard to resource or environmental costs.
C. Arden-Clarke's work on parts I and II of this review of the impacts of farming systems on the soil, was made possible by funding from the Eva Reckitt Trust Fund, the Augustine Trust, the Vegetarian Society and Planetary Initiatives. An initial draft of this paper was criticised by Drs. P. Newbould, D.A. Atkinson, H.H. Koepf and W. Adams, to whom the authors are grateful for their comments.
A.A.C. (1970). Modern Farming and the Soil Report of the Agricultural Advisory Council. H.M.S.O.; London.
Alexander, M. (1971). Microbial Ecology. J. Wiley & Sons; London.
Allison, F.E. (1973). Soil Organic Matter and its Role in Crop Production. Elsevier; London.
Arden-Clarke, C. & Hodges, R.D. (1987). The environmental effects of conventional and
organic/biological farming systems. l. Soil erosion, with special reference to Britain. Biological
Agriculture & Horticulture, 4, 309-357.
Atkinson, D.A. (1986). Personal Communication.
Baker, K. F. & Cook, R.J. (1974). Biological Control of Plant Pathogens. Freeman, San Francisco.
Balloni, W. & Favilli, F. (1987). Effects of agricultural practices on the physical, chemical and biological properties of soils: Part I--Effect of some agricultural practices on the biological soil fertility. In Scientific Basis for Soil Protection in the European Community (H. Barth & P. L'Hermite, eds.), pp.161-179. Elsevier; London.
Barney, P. A. ( 1987). The use of Trifolium repens, Trifolium subterraneum and Medicago lupulina as Overwintering leguminous green manures. Biological Agriculture & Horticulture, 4,225-234.
Bayley, R.W. (1970). Nitrogen and phosphate removal: methods and costs. Water Treatment & Examination, 19, 294.
Biederbeck,V.0.(1978).Soil organic sulphur and fertility .ln Soil Organic Matter(M.Schnitzer & S.U. Kahn, eds.), Developments in Soil Science, 8, pp.273-310. Elsevier; Amsterdam.
Boardman, J. (1983). Soil erosion at Albourne, West Sussex, England. Applied Geography, 3,
Bogovski, P. & Bogovski, S. (1981). Animal species in which N-nitroso compounds induce cancer. International Journal of Cancer, 27, 471-474.
Bolton, H., Elliott, L.F., Papendick, R.l. & Bezdicek, D.F. (1985). Soil microbial biomass and selected soil enzyme activities: effect of fertilization and cropping practices. Soil Biology & Biochemistry, 17, 297-302.
Boorman,L.A.&Fuller,R.M.(1981).The changing status of reed swamp in the Norfolk Broads. Journal of Applied Ecology, 18, 241-269.
B.O.S.C. (1985). British Organic Standards Committee Standards for Organic Produce.
Briggs, D.J. & Courtney, F. M. (1985). Agriculture and Environment. The Physical Geography of Temperate Agricultural Systems. Longman; London.
Burford, J.R., Greenland, D.J. & Pain, B.F. (1976). Effects of heavy dressings of slurry and inorganic fertilisers applied to grassland on the composition of drainage waters and the soil atmosphere. In Agriculture and Water Quality, M.A.F.F. Technical Bulletin No.32,432-443.
Bussler, W. (1974a). The importance of "balanced fertilisers" with 12 mineral nutrients for higher yields of adequate quality. In Fertilizers, Crop Quality and Economy (V.H. Fernandez ed.), pp.503-532. Elsevier; Amsterdam.
Bussler, W. (1974b). Experimental contributions to the problem of improving the nutritional quality of food plants. In Fertilizers, Crop Quality and Economy (V.H. Fernandez, ea.) pp.532-540. Elsevier; Amsterdam.
Cameron, K. C. & Wild, A. (1984). Potential aquifer pollution from nitrate leaching following the plowing of temporary grassland. Journal of environmental Quality, 13, 274-278.
Christensen, S. (1983). N2O emission from a soil under permanent grass: seasonal and diurnal fluctuations as influenced by manuring and fertilization. Soil Biology & Biochemistry, 15,
Chaney, K. & Swift, R.S. (1984). The influence of organic matter on aggregate stability in some British soils. Journal of Soil Science, 35, 223-230.
Church, B.M. (1975). Use of fertilizers in England and Wales, 1974. Rothamsted Experimental Station (R.E.S.) Report for 1974, part II, pp.195-199. R.E.S., Harpenden, Herts
Church, B.M. (1982). Use of fertilizers in England and Wales, 1981. Rothamsted Experimental Station Report for 1981, part II, pp. 123-128. R.E.S.; Harpenden, Herts.
Church, B.M. (1985). Use of fertilizers in England and Wales, 1984. Rothamsted Experimental Station Report for 1984, part II, pp.277-284. R.E.S.; Harpenden, Herts.
Clement, C.R. (1961). Benefit of leys--structural improvement or N reserves. Journal of the British Grassland Society, 16, 194-200.
Clement, C.R. & Williams, T.E. (1967). Leys and soil organic matter. II. The accumulation of N in soils under different leys. Journal of Agricultural Science, 69, 133-138.
Coleman, D.C., Cole, C.V. & Elliot, T.E. (1984). Decomposition, organic matter turnover, and nutrient dynamics in agroecosystems. In Agricultural Ecosystems: Unifying Concepts (R. Lowrance, B. R. Stinner & G.J. House, eds.), pp. 83- 104. John Wiley; Chichester.
Coleman, E.W. & Ridgway, R.L. (1983). Role of stress tolerance in integrated pest management. In Sustainable Food Systems (D. Knorr, ed.), pp. 124-142. A.V.I. Publishing Co.; Westport, Conn.
Cook, R.J. (1977). Management of the associated microbiota. In Plant Disease (J.G. Horsfall & E.B. Cowling, eds.), Vol. 1, pp. 145-166. Academic Press; New York.
Cook, R.J. (1981). Biological control of plant pathogens: Overview. In Biological Control in Crop Production (G.C. Papavizas, ed.), pp.23-44. Allanheld, Osmun; Ottawa.
Cook, R.J. (1986). Plant health and the sustainability of agriculture, with special reference to disease control by beneficial organisms. In The Role of Microorganisms in a Sustainable Agriculture (J.M. Lopez-Real & R.D. Hodges, eds.), pp.125-146. AB Academic Publishers; Berkhamstead.
Cooke, G.W. (1954). Recent advances in fertilizer placement. ill. Fertilizer placement in England. Journal of the Science of Food and Agriculture, 9, 429-440.
Cooke, G.W. (1967). The Control of Soil Fertility. English Language Book Society and Crosby Lockwood Staples London.
Cooke, G.W. (1976a;. A review of the affect of agriculture on the chemical composition and quality of surface and underground waters. In Agriculture and Water Quality. M.A.F.F. Technical Bulletin No. 32, pp.5-57.
Cooke, G.W. (1976b). The role of organic manures and legumes in crop production. In Energy Use in British Agriculture (D.M. Bather & C.l. Day, eds.). Reading University Agricultural Club, Reading.
Cooke, G.W. (1977a). The roles of organic manures and organic matter in managing soils for higher crop yields: a review of the experimental evidence. In Proceedings of the International Seminar on Soil Environment and Fertility Management in Intensive Agriculture, pp. 53-64. Society of the Science of Soil and Manure, Japan.
Cooke, G.W. (1977b). Waste of fertilizers. Philosophical Transactions of the Royal Society, B. 281, 231-241.
Cooke, G.W. (1979). Some priorities for British soil scien. Journal of Soil Science, SO, 187-213.
Cooke, G.W. (1982). Fertilising for Maximum Yields (3rd. edn.). Granada; London.
Cornforth, I.S. (1965). Soil aggregation. Rothamsted Experimental Station Report for 1964, p.49. R.E.S.; Harpenden, Herts.
Cowling, D.W. (1982). Biological nitrogen fixation and grassland production in the U.K. Philosophical Transactions of the Royal Society, B. 296, 397-404.
Cowling, D.W. & Jones, L.H.P. (1970). A deficiency in soil sulphur supplies for perennial ryegrass in England. Soil Science, 110, 346-354.
Cox, G.W. (1984). The linkage of inputs to outputs in agroecosystems. In Agricultural Ecosystems: Unifying Concepts (R. Lowrance, B.R. Stinner & G.J. House, eds.), pp.187-208. John Wiley; Chichester.
Currey, J.P. (1976). Some effects of animal manures on earthworms in grassland. Pedobiologia, 16, 425-438.
Davidescu, D. (1974). Chemical fertilizers and crop quality. In Fertilizers. Crop Quality and Economy (V.H. Fernandez, ed.), pp.1073-1104. Elsevier; Amsterdam.
Dean, R., Ellis, J.E., Rice, R.W. & Bement, R.E. (1975). Nutrient removal by cattle from a shortgrass prairie. Journal of Applied Ecology, 12, 25-29.
Deavin, A. (1978). Soil cover, soil organic matter, the rhizosphere and plant growth. In The Ecology of Cultivation. Proceedings of a European Seminar organized by the Federal German Council for Land Conservation, pp.21-34.
Department of the Environment (1984). Standing Technical Committee on Water Quality. Fourth Biennial Report. H.M.S.O.; London.
Dight, R.J.W. (1983). Environmental aspects of the use of organic farm wastes and sewage sludges. Fertiliser Society Proceedings, 222.
Dijk, T.A. van & Sturm, H. (1983). Fertiliser value of animal manures on the continent. Fertiliser Society Proceedings, 220.
Dijk, H. van (1971). Colloid chemical properties of humic matter. In Soil Biochemistry (A D. Maclaren & J. Skujins, eds.), pp.16-35. Marcel Dekker; New York.
Doebereiner, J. (1977). New opportunities for exploitation of biological nitrogen fixation as an alternative N source without environmental hazards. Ambio, 6, 174-177.
Draycott, A.P., Durrant, M.J. & Webb, D.J. (1978). Long-term effects of fertilizers at Broom's Barn, 1971-76. Rothamsted Experimental Station Report for 1977, part 11, pp. 15-30. R.E.S., Harpenden, Herts.
Drew, M.C. (1975). Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist, 75, 479-490.
Drew, M.C. (1976). The effect of the supply of mineral nutrients on root morphology, nutrient uptake and shoot growth in cereals. A.R.C. Letcombe Laboratory Annual Report for 1975, pp.63-73.
Dyke, G.C. & Barnard, A.J. (1976). Suppression of couch grass by Italian ryegrass and b road red clover undersown in barley and field beans. Journal of Agricultural Science, Cambridge, 87, 123-126.
Dyke, G.C., Patterson, H.D. & Barnes, T.W. (1977). The Woburn Long-term experiment on green manuring, 1946-67; results with barley. Rothamsted Experimental Station Report for 1976, part 11: 119-152. R.E.S.; Harpenden, Herts.
Eagle, D.J. (1975). ADAS ley fertility experiments. In Soil Physical Conditions and Crop Production. MAFF Technical Bulletin No. 29, pp.344-349. HMSO, London
Edwards, C.A. (1984). Changes in agricultural practice and their impact on soil organisms. In Agriculture and the Environment (D. Jenkins, ea.) pp.56-65. Proceedings of Institute of Terrestrial Ecology (ITE) Symposium, 13, Monks Wood Experimental Station.
Edwards, C.A. & Lofty, J.R. (1969). The influence of agricultural practice on soil microarthropod populations. In The Soil Ecosystem (J.G. Sheals, ed.), pp. 237-247. Systematics Association Publications, 8.
Edwards, C.A. & Lofty, J.R. (1971). Nematicides and the soil fauna. In Proceedings of the Sixth British Insecticides and Fungicides Conference, Brighton. Pp.158-166.
Edwards, C.A. & Lofty, J.R. (1977). Biology of Earthworms (2nd edn.). Chapman & Hall; London.
Edwards, C.A. & Lofty, J.R. (1980). Effects of earthworm inoculation upon the root growth of direct-drilled cereals. Journal of Applied Ecology, 17, 533-543.
Edwards, C.A. & Lofty, J.R. (1982). Nitrogenous fertilizers and earthworm populations in agricultural soils. Soil Biology & Biochemistry, 14, 515-521.
El-Fouly, M.M. (1976). The effect of N fertilizers on growth of cereals and the impact on disease. In Fertilizer Use and Plant Health. Proceedings of the 12th Colloquium of the International Potash Institute, pp.69-76. International Potash Institute, Worblaufen, Bem
Elliott, L.F. & Papendick, R.I. (1986). Crop residue management for improved soil productivity. In The Role of Microorganisms in a Sustainable Agriculture (J.M. Lopez-Real & R.D. Hodges eds.), pp.45-56. AB Academic Publishers; Berkhamstead.
Evans, R. & Nortcliff, S. (1978). Soil erosion in north Norfolk. Journal of Agricultural Science, Cambridge, 90, 185-192.
Fedoroff, N. (1987). The production potential of soils: Part I--sensitivity of principal soil types to the intensive agriculture of north-western Europe. In Scientific Basis for Soil Protection in the European Community (H. Barth & P. L`Hermite, eds.), pp.29-64 Elsevier, London
Floate, M.J.S. (1970). Mineralization of nitrogen and phosphorus from organic materials of plant and animal origin and its significance in the nutrient cycle in grazed upland and hill soils. Journal of the British Grassland Society, 25, 295-302.
Forman, D., Al-Dabbagh, S. & Doll, R. (1985). Nitrates, nitrites and gastric cancer in Britain. Nature, 313, 620-625.
Foster, S.S.D., Cripps, A.C. & Smith-Carrington, A. (1982). Nitrate leaching to groundwater. Philosophical Transactions of the Royal Society, B, 296, 477-489.
Fragstein, P. von & Vogtmann, H. (1983). Organic extracts for the treatment of rock powder fertilisers in biological agriculture. Biological Agriculture & Horticulture, 1, 169-180.
Fragstein, P. von, Pertl, W. & Vogtmann, H. (1986). Silicate rock powders. Qualitative and quantitative aspects. In The Importance of Biological Agriculture in a World of Diminishing Resources (H. Vogtmann, E. Boehncke & I. Fricke, eds.), pp.74-84. Verlagsgruppe Weiland, Hupp, Burkhard; W~tzenhausen.
Fraser, D.G. (1984). Effects of conventional and organic management practices on soil microbial populations and activities. Unpublished MSc Thesis, University of Nebraska
Friend, G. (1983). The potential of sustainable agriculture. In Sustainable Food .Systems (D. Knorr, ed.), pp.28-47. Ellis Horwood; Chichester.
Frissel, M.J. (1978). Relative importance of N, P and K. In Cychng of Mineral Nutrients in Agricultural Ecosystems (M.J. Frissel, ed.), pp.305-306. Elsevier; Amsterdam.
Garwood, E.A., Ryden, J.C. & Tyson, K.C. (1986). Nitrate loss through leaching and surface run-off from grassland: effects of water supply, soil type and management. In Nitrogen Flows in Intensive Grassland Systems (H.G. Van der Meer, J.C. Ryden & G.C. Ennick, eds.), pp.99-113. Martinus Nijhoff; Dordrecht.
Gerdemann, J.W. (1975). Vesicular-arbuscular mycorrhizae. In The Development and Function of Roots (J.G. Torrey & D.T. Clarkson, eds.), pp.575-591. Proceedings of the 3rd Cabot Symposium. Academic Press; London.
Green, F.H.W. (1973). Aspects of the changing environment: some factors affecting the aquatic environment in recent years. Journal of Environmental Management, 1, 377-391.
Haan, F. A.M. de (1987). Effects of agricultural practices on the physical, chemical and biological properties of soils: Part III--chemical degradation of soil as a result of the use of mineral fertilizers and pesticides: aspects of soil quality evaluation. In Scientific Basis for Soil Protection in the European Community (H. Barth & P. L'Hermite, eds.), pp.211-236. Elsevier; London.
Harper, C.S. (1955). Soluble salts in the soil. N.A.A.S. Quarterly Review, 28, 143-152.
Harris, R.F., Chesters, G. & Allen, O.N. (1966). Dynamics of soil aggregation. Advances in Agronomy, 18, 107-169.
Haworth, F. & Cleaver, T.J. (1965). Soil potassium and the growth of vegetable seedlings. II. Effect of K and NH4 salts on the growth and composition of seedlings. Journal of the Science of Food and Agriculture, 16, 600-604.
Hayman, D.S. (1975). Phosphorous cycling of soil microorganism and plant roots. In Soil Microbiology (N. Walker, ed.), pp.67-91. Halstead Press; New York and Wiley & Sons.
Henin, S. (1986). Water quality--the French problem. In Effects of Land Use on Freshwaters: Agriculture, Forestry, Mineral exploitation, Urbanisation (J.F. de L.G. Solbe, ed.), pp.267-282. Ellis Horwood; Chichester.
Herbert, J. (1977). Control of the nitrogen fertilization in intensive farming for minimizing water pollution. In Proceedings of the International Seminar on Soil environment and Fertility Management in Intensive Agriculture, pp.325-337. Society of the Science of Soil and Manure; Tokyo, Japan.
Hodges, R.D. (1977). Who needs granular inorganic fertilizers anyway? The case for biological agriculture. Proceedings of the International Conference on Granular Fertilizers and their Production, pp.224-244. The British Sulphur Corporation Ltd.; London.
Hodges, R. D. (1981). An agriculture for the future. In Biological Husbandry (B. Stonehouse, ed. ), pp.1-14. Butterworths; London.
Hodges, R.D. (1985). Agriculture, nitrates and health. Soil Association Revieu, September 1985, pp.16-18.
Hodges, R.D. & Scofield, A.M. (1983). Agricologenic disease. A review of the negative aspects of agricultural systems. Biological Agriculture & Horticulture, 1, 269-325.
Holden, A.V. (1976). The relative importance of agricultural fertilizers as a source of N and P in Loch Leven. In Agriculture and Water Quality, M.A.F.F. Technical Bulletin, 32, 306-314.
Holden, A.V. & Caines, L.A. (1974). Nutrient chemistry of Loch Leven, Kinross. Proceedings of the Royal Society of Edinburgh, 74, 101-121.
Holt, R.F., Timmons, D.R. & Latterell, J.J. (1970). Accumulation of phosphates in water. Journal of Agricultural & Food Chemistry, 18, 781-784.
Horne,B.(1973).Leys and soil fertility. I.Crop production. Experimental Husbandry, 23,86-103.
Huber, D.M. & Watson, R.D. (1974). Nitrogen form and plant disease. Annual Review of Phytopathology, 12, 139-165.
Imai H. (1977). The harmful effects of ammonium nitrogen on crop roots. In Proceedings of the International Seminar on Soil environment and Fertility Management in Intensive Agriculture, pp.634-640. Society of the Science of Soil and Manure. Tokyo; Japan.
Jacks, G.V. (1963). The biological nature of soil productivity. Soils & Fertilizers, 26, 147-150. Jenkinson, D.S. & Johnston, A.E. (1977). Soil Organic Matter in the Hoosfield Continuous Barley Experiment. Rothamsted Experimental Station Report for 1976, Part II, pp.87-102. R.E.S.; Harpenden, Herts.
Jenkyn, J.F. (1976). Nitrogen and lea diseases of spring barley. In Fertilizer Use and Plant Health. Proceedings of the 12th Colloquium of the International Potash Institute. Worblaufen, Bern.
Johnston, A.E. (1982). The effects of farming systems on the amount of soil organic matter and its effect on yield at Rothamsted and Woburn. In Proceedings of the Land Use Seminar on Soil
Degradation(D.Boels, D.B.Davies & A.E.Johnston,eds.),pp.107-131.Balkema,Rotterdam Johnston, A E (1986). Soil organic matter, effects on soils and crops, Soil Use & Management
Johnston, A.E. & Winham, W.N. (1980). The use of lime on agricultural soils. Fertiliser Society Proceedings, 189.
Kemp, A. (1971). The effects of potassium and nitrogen dressings on the mineral supply of grazing animals. In Potassium and Systems of Grassland Farming. Colloquium No. I, pp.79-92. The Potassium Institute; Henley-on-Thames.
Kiraly, Z. (1976). Plant disease resistance as influenced by biochemical effects of nutrients in fertilizers. In Fertilizer Use and Plant Health. Proceedings of the 12th Colloquium of the International Potash Institute, pp.33-46. International Potash Institute; Worblaufen, Bern.
Koepf. H.H. (1974). Organic management reduces leaching of nitrate. Compost Science 15 (Nov/Dec), 11-15.
Koepf,H.H.,Petterson,B.D.&Schaumann,W.(1976). Biodynamic Agriculture. An introduction. The Anthroposophic Press, New York.
Koepf, H.H. (1986). Personal communication.
Kononova, M.M. (1966). Soil Organic Matter. 2nd. English edn. Pergamon Press; Oxford.
Kurtz, L T (1970). The fate of applied nutrients in soils. Journal of Agricultural & Food Chemist/J,.
Larsen, S (1976). Phosphorus in past, present and future agriculture. Phosphorus in Agriculture
Lassen, R., Tjell, J.C. & Hansen, J.A. (1984). Phosphorus recovery from sewage for agriculture. Waste Management & Research, 2, 369-378.
Lockeretz, W., Keppler, R., Commoner, B., Gertler, M., Fast, S. & O'Leary, D. (1976). Organic and Conventional Crop Production in the Corn Belt: a Comparison of Economic Performance and Energy Use for Selected Farms. Report CBNS-AE-7. Center for the Biology of Natural Systems, Washington University; St. Louis, MO.
Lockeretz, W., Shearer, G., Sweeney, S., Kuepper, G., Wennu, D. & Kohl, D. (1980). Maize yields and soil nutrient levels with and without pesticides and standard commercial fertilizers. Agronomy Journal, 72, 65-72.
Lockhart, J.A.R. & Wiseman, A.J.L. (1983). Introduction to Crop Husbandry. 5th. edition. Pergamon; Oxford.
Long, E. (1985). Metal mayhem. Farmers Weekly, 22nd. November, 1985, pp.67-68.
Lovett, J.V. (1980). Annual field crops. In Perspectives in World Agriculture, Chapter 4, pp.91-122. Commonwealth Agricultural Bureaux; Farnham Royal.
Lunt, H.A. & Jacobson, G.M. (1944). The chemical composition of earthworm casts. Soil Science,58, 367-375.
McAllister, J.S.V. (1974). Resource management-farm effluents. In NW European Region Symposium on Intensive Agriculture and the environment, C.I.C.R.A., pp.71-79. An Foras Taluntais, Dublin.
McElroy, M.B., Wofsy, S.C. & Yung, Y.L. (1977). The nitrogen cycle: perturbations due to man and their impact on atmospheric N2O and O3. Philosophical Transactions of the Royal .Society, B,
McGrath, S.P., Brookes, P.C. & Giller, K.E. (1987). Long-term biological effects of metals after application of sewage sludge. Journal of the Science of Food & Agriculture, in press.
McRae, R.J. & Mehuys, G.R. (1987). Effects of green manuring in rotation with corn on the phys~cal properties of two Quebec soils. Biological Agriculture & Horticulture 4, 257-270.
Madge, D.S. (1981). Influence of agricultural practice on soil invertebrate animals. In Biological Husbandry (B. Stonehouse, ed.), pp.79-98. Butterworths; London.
M.A.F.F. (1976). Organic Manures. Ministry of Agriculture, Fisheries & Food Bulletin No. 210. H.M.S.O.; London.
Magee, P. N. (1982). Nitrogen as a potential health hazard. Philosophical Transactions of the Royal Society, B, 296, 543-550.
Maitland, P.S. (1984). The effects of eutrophication on aquatic wildlife. In Agriculture and the Environment(D. Jenkins, ed.), pp.101-108. Proceedings of the Institute of Terrestrial Ecology Symposium, 13. Monks Wood Experimental Station.
Marschner. H. (1986). Mineral Nutrition in Higher Plants. Academic Press; London.
Marshall, V.G. (1977). Effects of manures and fertilizers on soil fauna: a review. Special Publications, No. 3. Commonwealth Bureau of Soils; Harpenden, Herts.
Martyniuk, S. & Wagner, G.M. (1978). Quantitative and qualitative examinations of soil microflora associated with different management systems. Soil Science, 125, 343-350.
Mattingly, G.E.G. (1974). The Woburn organic manuring experiment. I. Design, crop yields and nutrient balance, 1964-72. Rothamsted Experimental Station Report for 1973, Part II, pp.98-133. R.E.S., Harpenden, Herts.
Mattingly, G.E.G., Chater, M. & Johnston, A.E. (1975). Experiments made on Stackyard Field, Woburn, 1867-1974. III. Effects of NPK fertilisers and FYM on soil C, N and organic P. Rothamsted Experimental Station Report for 1974, part II, pp.61-77. R.E.S.; Harpenden, Herts.
Mengel, K. & Kirkby, E.A. (1982). Principles of Plant Nutrition, 3rd. edn. International Potash Institute, Worblaufen-Bern.
Mortvedt, J.J. (1986). Methods of applying micronutrient fertilizers to correct deficiencies of crops. Outlook on Agriculture, 15, 135-140.
Mosse, B. (1986). Mycorrhiza in a sustainable agriculture. Biological Agriculture & Horticulture, 3 191 -209.
Muider, D. (1953). Les elements mineur en culture fruitiere. In I Convegno di Frutticoltura, Montana di St. Vincent, pp.188-198.
N.C.C. (1984). Acid deposition and its implications for nature conservation in Britain. Focus on Nature Conservation, 7. Nature Conservancy Council, Shrewsbury.
Newbould, P. (1982). Losses and accumulation of organic matter in soils. In Proceedings of the Land Use Seminar on Soil Degradation (D. Boels, D.B. Davies & A.E. Johnston, eds.), pp.107-131. Balkema; Rotterdam.
Oakes, D.B. et al (1981). The effects of farming practices on groundwater quality in the U.K. Science of the Total Environment, 21, 17-30.
Ott, P., Hansen, S. & Vogtmann, H. (1983) Nitrates in relation to composting and use of farmyard manures. In Environmentally Sound Agriculture (W. Lockeretz, ed.), pp.145-154. Praeger; New York.
Parr, J.F., Papendick, R.I. & Youngberg, I.G. (1983). Organic farming in the US: principles and perspectives. Agro-Ecosystems, 8, 183-201.
Parsons, J.W. (1984). Green manuring. Outlook on Agriculture, 13, 20-23.
Patrick, Z.A. & Tousson, T.A. (1965). Plant residues and organic amendments in relation to biological control. In Ecology of Soil-Borne Plant Pathogens ( K. F. Baker & W.C. Snyder, eds. ), pp.440457. John Murrray; London.
Patriquin, D.G. (1984). Biological agriculture: an affordable alternative. The Right to Food Conference, Concordia University, Montreal, May 1984.
Phillips, V.R. (1986). Remedies to problems caused by agriculture-the engineering solution. In effects of Land Use on Fresh waters Agriculture, Forestry, Mineral Exploitation, Urbanisation (J.F. de L.G. Solbe, ed.), pp.315-328. Ellis Horwood; Chichester.
Pimentel, D. ed. (1980). Handbook of energy Utilization in Agriculture. CRC Press; Boca Raton, Florida.
Pimentel, D., Terhune, E.C., Dyson-Hudson, R., Rocherau, S., Samis, R., Smith, E.A., Denman, D., Reifschneider, D. & Shepard, M. (1976). Land degradation: effects on food and energy reserves. Science, 194 149-155.
Power, J.F. & Doran, J.W. (i984). Nitrogen use in organic farming. In: Nitrogen in Crop Production (R. Hauck, ed. ), pp.585-598. American Society of Agronomy; Madison, Wisconsin.
Pratt, P.F., David, S. & Sharpless, R.G. (1976) A four year trial with animal manures. Hilgardia, 44, 99-112.
Rankin, J.D. & Taylor, R.J. (1969). A study of some disease hazards which could be associated with the system of applying cattle slurry to pasture. Veterinary Record, 85, 578-581.
R.C.E.P. (1979). Agriculture and Pollution. The Seventh Report of the Royal Commission on Environmental Pollution. Cmnd. 7644. H.M.S.O.; London.
Read, D.J., Francis, R. & Finlay, R.D. (1985). Mycorrhizal mycelia and nutrient cycling in plant communities. In Ecological Interactions m Soil, Plants, Microbes and Animals (A.H. Fitter, D. Atkinson, D.J. Read & M.B. Usher, eds.). British Ecological Society Special Publication, 4, 193-217. Blackwell; Oxford.
Reichle, D.E. (1977). The role of soil invertebrates in nutrient cycling. In Soil Organisms as Components of Ecosystems (W. Lohm & T. Persson, eds.), pp.145-156. Ecological Bulletins (Stockholm), vol. 25.
Rosc,oe, B. (1960) The distribution and condition of soil phosphate under old permanent pasture.
Roy, B B. & Thomas, B. (1951). The phosphate reserves in some old permanent pasture plots. Empire Journal of experimental Agriculture, 19, 175-184.
Russell, E.W. (1973). Soil Conditions and Plant Growth. Longman; London.
Russell, E.W. (1977). The role of organic matter in soil fertility. Philosophical Transactions of the Royal Society, B, 281, 209-219.
Ryden, J.C., Ball, P.R. & Garwood, E.A., (1984). Nitrate leaching from grassland. Nature, 311,
Ryden, J C, Syers, J K. & Harris, R.F. (1973). Phosphorus in run-off and streams. Advances in
Salter, P.J. & Williams, J.B. (1983). The effect of farmyard manure on the moisture characteristics of a sandy loam soil. Journal of Soil Science, 14, 73-81.
Salter, P.J. & Williams, J.B. (1969). The moisture characteristics of some Rothamsted, Woburn and Saxmundham soils. Journal of Agricultural Science, Cambridge, 73, 155-158
Sanders F.E. & Tinker, P.B. (1973). Phosphate flow into mycorrhizal roots. Pesticide Science, 4,
Schutte, K H (1964). The Biology of Trace Elements. Their Role in Nutrition. J.B. Lippincott;
Scott Russell, R. (1977). Plant Root Systems: Their Function and Interaction with the Soil. McGraw-H~II; London.
Scullion, J. & Ramshaw, G.A. (1987). Effects of manurial treatments on earthworm activity in grassland. Biological Agriculture & Horticulture, 4, 271-281.
Seastedth, T R. & Crossley, D.A. (1984). The influence of arthropods on ecosystems. Bioscience,
Shiel, R.S. & Rimmer, D.L. (1984). Changes in soil structure and biological activity on some meadow hay plots at Cockle Park, Northumberland. Plant & Soil, 76, 349-356.
Shipton, P.J. (1979). Experimental evidence for the effect on soil-borne diseases of changes in techniques of crop and soil cultivation. In Soil-Borne Plant Pathogens (B. Schippers & W. Gams, eds.), pp.385-397. Academic Press, London
Sluijsmans, C.M.J. & Kolenbrander, G.J. (1977). The significance of animal manures as a source of nitrogen m soils. In Proceedings of the later national Seminar on Soil environment and Fertility Management in Intensive Agriculture, pp.403418. Society for the Science of Soil and Manure. Tokyo; Japan.
Smith, G.E. (1942). Sanborn Field: fifty years of field experiments with crop rotations, manure and fertilizers. Missouri Agricultural experimental Station Bulletin, 458
Smith, K.A. & Unwin, R.J. (1983). Fertiliser value of organic manures in the U.K. Fertiliser Society Proceedings, 221.
Stewart, V.I. & Salih, R.O. (1981). Priorities for soil use in temperate climates. In Biological Husbandry (B. Stonehouse, ed.), pp.19-37. Butterworths; London.
Stojkovska, A. & Cooke, G.W. (1958). Micronutrients in fertilizers. Chemistry & Industry, 1368.
Stringer, A. & Lyons, C.H. (1974). Effect of benomyl and thiophanate methyl on earthworm populations in apple orchards. Pesticide Science, 5, 189-196.
Stroo, H.F. & Alexander, M. (1986). Role of soil organic matter in the effect of acid rain on nitrogen mineralisation. Soil Science Society of America Journal, 50, 1219-1223.
Suzuki, T., Tsuru, S., Fukushi, S. & Kobayashi, H. (1971). Fate and behaviour of animal wastes in an agricultural ecosystem. In Proceedings of the International Seminar on Soil environment and Fertility Management in Intensive Agriculture, pp.669-676. Society of the Science of Soil and Manure. Tokyo; Japan.
Syers, J.K. & Springett, J.A. (1984). Earthworms and soil fertility. P/ant & Soil, 76, 93-104.
Takahashi, T., Kushizaki, M. & Ogata, T. (1977). Mineral composition of Japanese grassland under heavy use of fertilizers--A review of two recent cooperative works. In Proceedings of the International Seminar on Soil environment and Fertility Management in Intensive Agriculture, pp. l 18-125. Society of the Science of Soil & Manure. Tokyo; Japan.
Tinker, P.B. (1984). The role of microorganisms in mediating and facilitating the uptake of plant nutrients from the soil. Plant & Soil, 76, 77-91.
Ulbricht, T.L.V. (1981). Feeding the world. In Biological Husbandry (B. Stonehouse, ed.), pp.331-338. Butterworths; London.
Unger, P.W. (1978). Straw-mulch rate effect on soil water storage and sorghum yield. Soil Science Society of America Journal, 42, 486-491.
U.S.D.A. (1980). Report and Recommendations on Organic Farming. U.S. Department of Agriculture study on organic farming. U.S. Government Printing Office; Washington, D.C.
Vereijken, P. (1986). Maintenance of soil fertility on the Biodynamic farm of Nagele. In The Importance of Biological Agriculture in a World of Diminishing Resources (H. Vogtmann, E. Boehncke & I. Fricke, eds.), pp.23-30. Verlagsgruppen Weiland, Hupp, Burkhard; Witzenhausen
Vetter, H. & Steffens, G. (1981). Leaching of nitrogen after the spreading of slurry. In Nitrogen Losses and Surface Run-off, pp.251-271. ECSC, ECC, EAEC: Brussels.
Vine, A. & Bateman, D. (1981). Organic farming system in England and Wales: practice, performance and implications. University College of Wales; Aberystwyth.
Voisin, A. (1965). Fertilizer Application: Soil, Grass and Animal. Crosby; London.
Vogtmann, H. & Biedermann, R. (1985).The nitrate story--no end in sight. Nutrition & Health,3, 217-239.
Voo rburg, J. H. (1983). Utilisation of organic wastes as a fertiliser. Fertiliser Society Proceedings, 219.
Wadleigh, C.H. (1968). Waste in relation to agriculture and forestry. U.S. Department of Agriculture Miscellaneous Publications, 1065.
Warren, R.G., Johnston, A.E. & D'Arifat, J.M. (1965). Organic manures and soil organic matter. Rothamsted Experimental Report for 1964, pp.4047. R.E.S.; Harpenden, Herts.
Watanabe, H. (1975). On the amount of cast production by the megascolecid earthworm, Pheretima hypeiensis. Pedobiologia, 15, 20-28.
Weaver, J.E. (1926). Root Development of Field Crops. McGraw-Hill; London.
Webber, J. (1975). The influence of bulky organic materials and soil conditioners on soil properties and crop yields. In Soil Physical Conditions and Crop Production. M.A.F.F. Technical Bulletin, No.29, 406-416
White, R.J. (1983). Nitrate in British waters. Aqua, 2, 51-57.
Whitney, P.J. (1976). Microbial Plant Pathology. Hutchinson, London.
Widdowson, F.V. & Penny, A. (1973). Yields and N. P and K contents of the crops grown in the Rothamsted Reference Experiment, 1956-70. Rothamsted Experimental Station Report for 1972, part 11, pp. l l 1-130. R.E.S.; Harpenden, Herts.
Widdowson, F.V., Penny, A. & Hewitt, M.V. (1982). Results from the Woburn Reference Experiment. 111. Yields of the crops and recoveries of N. P. K and Mg from manures and soil, 1975-79. Rothamsted Experimental Station Report for 1981, part 11, pp.5-21. R.E.S.; Harpenden, Herts.
Wiersum, L.K. (1962). Uptake of N and P in relation to soil structure and nutrient mobility. Plant & Soil, 16, 62-70.
Wild, A. & Cameron, K.C. (1980). Soil nitrogen and nitrate leaching. In Soils and Agriculture (P.B. Tinker, ed.), C.R.A.C. Vol.ll, pp.43-63, Blackwell Scientific; Oxford.
Williams, C.H. & Lipsett, J. (1980). The build-up of available K under subterranean clover pastures on a podzolic soil. Australian Journal of Agricultural Research, 11, 473.
Williams, J.H. & Coker, E.G. (1981). Phosphorus in sewage sludge and its behaviour in soil. In Phosphorus in Sewage Sludge and Animal Waste Slurries (T.W.G. Hucker, & G. Catroux, eds.), pp.291-302. Reidel; Dordrecht.
Williams, R.J.B. (1971). Relationships between the composition of soils and physical measurements made on them. Rothamsted Experimental Station Report for 1970, part 11, pp. 5-35. R.E.S., Harpenden,
Williams, R.J.B. (1973). Changes in the nutrient reserves of the Rothamsted and Woburn Reference Experiment Soils. Rothamsted Experimental Station Report for 1972, part 11, pp.86-101. R.E.S.; Harpenden, Herts.
Williams, R.J.B. (1978). Effects of management and manuring on physical properties of some
Rothamsted and Woburn soils. Rothamsted Experimental Station Report for 1977 part 11
Williams, R.J.B., Stojkovska, A., Cooke, G.W. & Widdowson, F.V. (1960). Effects of fertilizers and FYM on the Cu, Mn, Mo and Zn removed by arable crops at Rothamsted. Journal of the Sc/ence of Food & Agriculture 11, 570-575
Willis, W O. & Evans, C. E. (197i). Our soil is valuable. Journal of Soil & Water Conservation, 32,
Wilson, S.J. & Cooke, R.U. (1980). Wind erosion. In Soil Erosion (M.J. Kirby & R.P.C. Morgan eds.), pp.217-251. John Wlley; London.
Witter, E. (1986). The fate of nitrogen during high temperature composting of sewage sludge straw mixtures. Ph.D. Thes~s; University of London, Wye College.
Woodward, L. & Burge, P. eds. (1982). Green Manures. Elm Farm Research Centre, Newbury
Wright, M.A. (1979). Effect of benomyl and some other systemic fungicides on earthworms Annals of Applied Biology, 87, 520-524.
Young, C.P. (1986). Nitrate in groundwater and the effects of ploughing on release of nitrates In effects of Land Use on Freshwaters: Agriculture, Forestry, Mineral exploitation, Urbanisation (J.F. de L.G. Solbe, ed.), pp.221-237. Ellis Horwood; Chichester.
Young, C.P. & Gray, E.M. (1978). Nitrate in Groundwater. Technical Report No.69. Water Research Centre; Medmenham.
(Received 4th March, 1987; accepted 26th January 1988).
Copyright © 19-- Name of Copyright Holder. Reprinted with permission. All rights reserved.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: eapinfo@macdonald.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster