JPR Index | Virtual Library | Magazine Rack | Search
By Doria Mueller-Beilschmidt
When the light-stable pyrethroids were introduced in the late 1970s, many important agricultural and public health pests had developed resistance to widely used insecticides. Pest resurgence and secondary pest outbreaks were a common occurrence wherever insecticides were being used.' Consequently, the appearance of the synthetic pyrethroids (SPs) brought hope of a fresh start.2 Some even believed them to be the "silver bullet" for future pest control.3
The previous articles in this series on SPs (JPR 10(2):41-44 and JPR 10(3):32-37) examined the development, chemistry, and economics of synthetic pyrethroids, as well as their effects on human and environmental health. This article looks at the development of resistance and secondary pest outbreaks following the use of pyrethroids to control agricultural insect pests and disease vectors. It considers two examples in depth, cotton insect pests and mosquitoes, and focuses on the persistent second and third generation SPs (for example, deltamethrin, cypermethrin, permethrin, and fenvalerate). SPs have been intensively used now for over ten years. What does their track record look like? Do SPs offer conventional agriculture and public health long-term cost-effective control? Or does heavy dependence on SPs, as their predecessors, lead to a chemical crisis?
Light-stable SPs are persistent, broad spectrum, and potent insecticides. Extremely small doses are sufficient to control many major pests. For example, DDT and toxaphene are applied at the rate of 1-2 kilograms per hectare (kg/ha), whereas SPs are applied at rates of 0.1-0.2 kg/ha At the time of its introduction, deltamethrin was one of the most potent insecticide on the market; it is over 100 times more acutely toxic than DDT to many primary pests.4
Shortly after their introduction, a couple of insightful people with good memories sent out words of caution about overuse of the light-stable SPs.4 e; As predicted, within only a couple years of their introduction problems increased with pest resurgence, secondary pest outbreaks, and resistance in association with SP use. There are now over 40 species of Insects pests known to have developed resistance to SPs (see Table 1).
Because DDT and SPs have the same mode of action in insects, resistance to one is likely to convey resistance to the other. Their common resistance mechanism is referred to as "knockdown resistance" (kdr) or target-site insensitivity. Kdr has been shown to be the most important of several SP resistance mechanisms in insect species. The genes that convey kdr are recessive, which means that they are only expressed when homozygous (two copies of the same gene present in one individual). Recessive genes can persist at low levers in a population without being detected even If no selection pressure is present. For example, although DDT had not been used to control house flies for over twenty years, the intensive use of SPs was able to select for kdr and to make SP use in effective within a very short period of time.4
The first cases of cross-resistance were documented in the mid-seventies in house flies in Europe and resistant house flies have since been found in Canada.4 Similar resistance problems were found in ticks in Australia by the fate 1970s. The diamondback moth, a serious pest in many vegetable crops which is resistant to DDT, has shown the following levers of resistance to some SPs: permethrin (110-fold),* cypermethrin (894 fold), deltamethrin (2235-fold), and fenvalerate (2880 fold).6
The legacy left behind by DDT is proving to be a shadow on the use of SPs. In 1980, at least 229 insect pests had documented resistance to DDT:, all of which have the potential of conveying cross-resistance to the SPs. As two researchers 6 put it, "the development of resistance to DDT by a particular pest some 30 years ego might be expected to repeat itself in the development of resistance to SPs. SPs should never have been used as the single available compound, and never on a massive scale."
The World Health Organization (WHO) of the United Nations encountered serious problems with resistance in their early attempts to use SPs to control anopheline mosquitoes (the mosquitoes that carry malaria). These early outbreaks of resistance were most likely due to cross-resistance conveyed by the widespread use of DDT.6 Of the 50 arthropod vectors (animal species that transmit a disease) recorded, to have resistance to insecticides, 49 have resistance to DDT and 11 of these are important malaria vectors. So far, 10 arthropod vectors have been found to be resistant to the SPs.7 Nevertheless, DDT is still WHO's insecticide of choice. The next most widely used insecticides are fenitrothion and deltamethrin.89
According to WHO, vector resistance to insecticides is the most outstanding technical problem impeding the development of vector control programs. In Latin America, despite an almost tenfold increase in insecticide spraying from 1984 to 1987, there has been a sevenfold increase in reported cases of malaria. Interestingly, Ecuador, Bolivia, Colombia, and Guatemala are increasingly using SPs"mostly deltamethrin, in their vector control programs.9
Yet, some never seem to learn. In 1988, over 8 million pounds of (100 percent and 75 percent active ingredient) DDT, and about 50 thousand pounds of (2.5 percent active ingredient) deltamethrin were used in 21 Latin America countries to control malaria vectors, almost twice as much as was used in 1987.9 Recently, the World Bank approved a malaria control project in the Amazon region of Brazil which provides 54 million dollars over a five-year-period for the purchase of DDT and deltamethrin to be used inside dwellings to control mosquitoes.'°
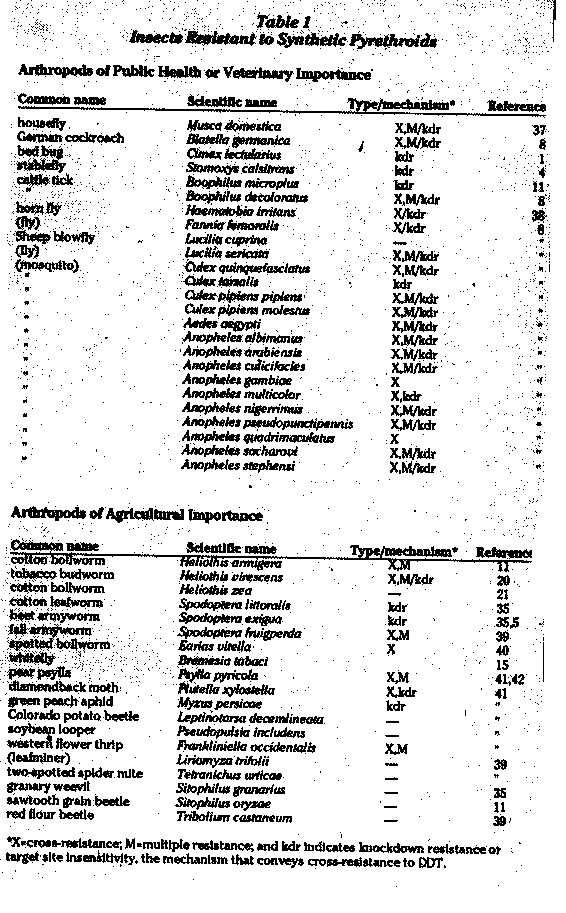
SPs were quickly adopted in cotton pest management throughout the world to replace the chlorinated hydrocarbons, such as DDT and toxaphene, and organophosphate insecticides. However, SPs do not control most plant sucking insects, such as spider mites (Acari: Tetranychidae), aphids (Homoptera Aphididae), and whiteflies (Homoptera: Aleyrodidae). They are more likely to cause increases in their populations or secondary pest outbreaks." Outbreaks in populations of whitefly on -cotton in India, Imperial Valley (California), and Thailand are believed to be associated with the introduction of SPs.'2'3
These secondary pest outbreaks of plant sucking pests are believed to be partially due to hormolygosis (see sidebar, p. 34).'4'5 Also, high applications of nitrogenous fertilizers increase the tolerance of plant sucking insect pests to SPs." 16
``By 1987, extensive resistance in Heliothis armigera to SPs (164 to 3QO-fold) was apparent. This was accompanied by secondary outbreaks of the whitefly and spider mites. Cotton production dropped from 608 kg/ha in 1984/85 to 263 kg/ha in 1987/88 .... "
The first documented case of high lever (many-fold) resistance to SPs was in Australia in 1982-83. Significant losses of cotton, sorghum and soybeans resulted because of the failure of SPs to control the cotton bollworm, Heliothis armigera. DDT resistance was prevalent throughout Australia s cotton growing area during the mid-seventies. Strains from the field were found to be both resistant and tolerant to SPs and DDT.
In the southern U.S. cotton growing region, SP resistance was found in some tobacco budworm (Heliothis virescens) populations from the time SPs were first used, although the first field failures (the application of an insecticide to a crop that does not lead to pest mortality) were reported in 1985. Tobacco budworm resistance extended to all SPs tested.20 More recently, there have been signs of resistance development in the bollworm to SPs in the southern U.S.; this raises an alarm since there are a lot more bollworms than tobacco budworms.21 In the Imperial Valley of California, tobacco budworm resistance was first seen in 1980 along with severe outbreaks of whitefly which were associated with the introduction of SPs (see Figure 2).22
The most recent and devastating example of how SP use and resistance to SPs can cause problems occurred in Andhra Pradesh in India during the 1987~8 cotton growing~season. SPs were introduced in 1980. Cotton- production used high levers of fertilizer, irrigation, and up to 30 insecticide treatments per season. The insecticides amounted to 30-40% of total production costs and at least one third of the treatments were SPs.23 By 198788, extensive resistance in Heliothis armigera to SPs (164- to 300 fold) was apparent. This was accompanied by secondary outbreaks of the whitefly and spider mites.24 Cotton production dropped from 608 kg/ha in 1984/85 to 263 kg/ha in 1987/88, which caused the economic ruin of dozens of small farmers in that region. Out of despair, many of the farmers and their spouses committed suicide by ingesting pesticides.24.25
Prior to the 1970s, resistance was probably was an overall benefit to industry because it opened up markets to new insecticides and increased sales. Since then, it has become more difficult and expensive to get a product developed, registered, produced, and marketed. Resistance shows up much more quickly for each new class of pesticides. The number of species resistant to DDT and methoxychlor doubled every 6.3 years, whereas the doubling time for the SPs was only two years.
As a result, shortly after the first signs of SP resistance were seen in 1979, IC! approached other major SP manufacturers to form the Pyrethroid Efficacy Group (PEG). PEG's approach focuses on 1) monitoring the development of resistance around the world and offering interpretation and publication of the results; 2) influencing work clone and pronouncements made on the subject of resistance; 3) making recommendations to delay onset or increases in resistance; and 4) countering unsubstantiated rumors of resistance which could "damage registration or product life or image.
In 1984, a larger international body was formed by the International Group of National Associations of Manufacturers of Agrochemical Products (GIFAP) called the Insecticide Resistance Action Committee IRAC). PEG is an independent sub-group of IRAC.27
The strategy allows a single nationwide SP treatment on cotton only to control the cotton leafworm (Spodoptera litoralis). Nevertheless, SP resistance appeared in ]985, and has been increasing since 1986.
PEG and IRAC have both been very involved in the management of resistance developments around the world, especially in cotton, its most lucrative market. They are also actively promoting the idea of insecticide resistance management (IRM) to try and prolong the rives of their products.2627
The goal of IRM is to conserve susceptibility. It can be either curative or preventative.28 Governments, the chemical manufacturers, growers, and researchers are involved. For example, the Egyptian government was determined to preserve the efficacy of SPs. Therefore, a mandatory IRM strategy was instituted for SP use on cotton in 1978. The strategy allows a single nationwide SP treatment on cotton only to control the cotton leafworm (Spodoptera littoralis). Nevertheless, SP resistance appeared in 1985, and has been increasing since 1986.29 In Australia, the IRM technique is curative, and based on an "SP window." SP use is limited to a critical 3~day period in the cotton season during which no more than three SP sprays are allowed to control Heliothis armigera. During the rest of the season, other insecticides with different modes of action (mostly organophosphates) are used: Despite virtually total compliance in the affected cotton growing areas, resistance is still increasing and field failures have been reported.2829
In the cotton growing area of the southern U.S., management practices to delay SP resistance in the tobacco budworm have been used since 1986. SP treatments are limited to a sixty day period in July and August. Other insecticides are used during other periods. The strategy includes close monitoring of tobacco budworm populations.29 Though 1987 and 1988 went well with almost no field failures, it was most likely due to low tobacco budworm populations. In 1989, the levers of resistance to SPs in tobacco budworm increased, causing control difficulties and, in some cases, economically unacceptable losses.30
California has its own approach to IRM in cotton: SPs are not used on cotton. The University of California's statewide IPM program advises California cotton growers not to use SPs because of secondary pest outbreaks, such as mites, and also because of resistance.31 In the San Joaquin Valley, where 95% of California's and 10% of the U.S. cotton is grown, most cotton growers do not use SPs. The same is not true in the Imperial Valley where growers are fighting a losing battle with resistance and applying insecticides including SPs) up to 20 times per season.
Is IRM working? Dr. Roman Sawicki;* one of the leading IRM scientists, made the following closing comment in a letter: "Last but not least, you will note from the lest paper with Denholm that I am not very optimistic about the success of insecticide resistance management, even in the most advanced and prosperous countries.33
It is important to remember that resistance is not only an economic problem. It also has major human and environmental health implications. When resistance occurs, pesticides are used at increasingly higher doses, mixtures of pesticides (with the potential of synergistic effects) are used, and the use of broad spectrum pesticides increases.36

1. Metcalf, R 1987. Benefit and risk considerations In the use of Insecticides. Robert van den Bosch Memorial Lecture, Berkeley, California.
2. Casida, John E. 1980. Pyrethrum flowers and pyrethroid insecticides. Envin Health Perspec. 34:189-202.
3. Phililps, 1R, J.B. Graves, and RC. Litrell. 1989. Insecticide resistance management. In The pyrethroid Insecticides: A scientific advance for human welfare. The Pyrethroids Efficacy Group, Proceedings of the 1989 Annual Meeting of the AAAS, San Francisco, January 19.
4. Elliot, M., N.F. Jan - , and C. Potter. 1978. The future of pyrethroids In Insect control. Ann. Rev Entomol 23.744~69.
5. Sparks, Thomas C. 1981. Development of Insecticide resistance In Heliothis zea and Heliothis virescens In North America. Entomol. Soc. Amer. Bul 27(3):186-192.
6. Miller, T.A. and V.L Salgado. 1985. The mode of action of pyrethroid on Insects. In J .P. Leahy (ed.) The pyrethroid insecticides, pp. 43-97. London, U.K.: Taylor & Francis.
7. White, G.B. 1989. Malaria In Geographical distribution of arthropod-borne diseases and their principal vectors, pp.7-9. Geneva, Switzerland: World Health Organization, Vector Biology and Control Division.
8. WHO Expert Committee on Vector Biology and Control. 1986. Resistance of vectors and reservoirs of disease to pesticides. Technical Report Series 737. Geneva, Switzerland: World Health Organization Dr. Sawicki died of a terminal illness only a few months after he wrote this letter. .
9. Pan American Health Organization. 1988. Status of Malaria Programs In the Americas. XXXVI Report. Geneva, Switzerland: World Health Organization.
10. Treakle, Kay. 1989. The disease of development: DDT threatens the Amazon. Greenpeace (November/December): 12-13.
11. Herve, JJ. 1985. Agricultural public health and animal usage. In J.P. Leahy (ed.) The pyrethroid insecticides, pp. 343~425. London, U.K.: Taylor Francis.
12. Jayaraj, S. et al. 1986. Studies on the outbreak of whitefly, Bremisia tabaci) (Gennadius) on cotton In Tamil Nadu. In S. Jayaraj (ed.) Resurgence of sucking pests. Proc. Nati. Symp. Coimbatore, India Tamil Nadu University.
13. Johnson, M.W., et al. 1982. Whiteflies cause problems for southern growers Calif. Agric. 36(g/10):24-26.
14. Dittrich, V. 1987. Resistance and hormolygosis as driving forces behind pest outbreaks. In KJ. Brent and R.K. Atkin (eds.) Rational pesticide use, pp. 169-181. Cambridge, U.K: Cambridge University Press.
15. Dittrich, V., S.O. Hassan, G.H. Ernst 1985. Sudanese cotton and the whitefly A case study of the emergence of a new primary pest. Crop Protection 4(2):161-176.
16. Reddy, A.S. 1987. Management of whitefly In cotton. Unpublished. Lam, India: Regional Agricultural Research Station.
17. Sawicki, RM. 1985. Report on a visit to Australia, January-February 1985, to Investigate Insecticide resistance In Heliothis armigera (Hubner). Unpublished, Harpenden, Herts, U.K.: Rothhamsted Experimental Station.
18. Daly, J.C. and DA Murray. 1988. Evolution of resistance to pyrethroids In Heliothis armigera (Huebner) (Lepidoptera: Noctuidae) In Australia. J. &on Entomol 81(4):984-988.
19. Gunning, RV. et al. 1984. pyrethroid resistance In Heliothis armigera (Huebner) (Lepidoptera: Noctuidae) In Australia. J. Econ Entomol. 77:1283-1287.
20. Leonard, B.R et al. 1988. Variation In resistance of field populations of tobacco budworm and bollworm (Lepidoptera: Noctuidae) to selected Insecticides. 1 Econ Entomol. 81(6):1521-1528.
21. NOR-AM Chemical Company. 1990. Outwitting pyrethroid resistance. Agrichemical Age (June): 12:14-15.
22. Martinez-Carrillo, J.L and H.T. Reynolds. 1983. Dosage-mortality studies with pyrethroids and other Insecticides on the tobacco budworm Lepidoptera Noctuidae) from the Imperial Valley, California J. Econ Entomol. 76:983-986.
23. Mehrotra, K.N. 1988. Crisis In cotton cultivation. Unpublished. Division of Entomology, Indian Agricultural Research Institute.
24. Sawicki, RM. 1988. Report on the control of cotton pests In central and southern India Unpublished. London, U.K: The Royal Society.
25. Menon, Amarnath K. 1988. Death harvest: Crop failure leads to suicides. India Today March 31, pp. 19-20.
26. Ruscoe, C.N.E. 1987. Pesticide resistance Strategies and co~operation In the agrochemical Industry. In KJ. Brent and RK. Atkin (eds.) Rational pesticide use, pp. 197 208. Cambridge, U.K: Cambridge University Press.
27. Jackson. GJ. 1986. Insecticide resistance What is Industry doing about It?. In Proceedings of the British Crop Protection Conference-Pests and Diseases . Vol. ll., pp. 943-949. Croydon, London, U.K: British Crop Protection Council.
28. Sawicki, RM., 1. Denholm. 1987. Management of resistance to pesticides in cotton pests. Tropical Pest Managem. 33(4):262272.
29. Sawicki, RM. 1989. Current Insecticide management practices In cotton around the world: Short term successes or templates for the future? Pesticide Science 26:401-410.
30. Graves, J.B. et d. 1990. Status d pyrethroid resistance In tobacco budworm In Louisiana. Unpublished. Baton Rouge, LA Louisiana Agricultural Experiment Station, LSU Agriculture Center.
31. Zalom, F., director, Statewide IPM Project University of California, Davis. Personal communication. 1990.
32. Baker, Brian P. 1988. Pest control In the public Interest: Crop protection In California J. Environ Law and Policy8(1):3171.
33. Sawicki, R, Rothhamsted Experiment Station, U.K Personal communication. 1990.
34. Gour, T.B. 1986. Factors Influencing whitefly outbreaks: A review. In S. Jayaraj (ed.) Resurgence of sucking pests, Proc. Natl. Symp. Coimbatore, India Tamil Nadu University.
35. Briggs, G.G., M. Elliot, and N.F. Janes. 1983. Present statue and future prospects for synthetic pyrethroids. In N. Tacahashi, H. Yoshioda, T. Misato, and S. Matsunaka (eds.) Pesticide Chemistry: Human welfare and the environment, Vol Z Natural products, pp. 157-164.
36. Knight, A.L. and G.W. Norton. 1989. Economics of agricultural pesticide resistance In arthropods. Ann. Rev. Entomol. 34:293-313.
37. Sawicki, RM. et al. 1984. Factors affecting resistance to Insecticides In house flies, Musca domestica L (Diptera Muscidae). U. Close linkage on autosome 2 between an esterase and resistance to trichiorfon and pyrethroids. Bull. Entomol Res. 74:197-206.
38. Roush, RT. et ad. 1986. Inheritance and effective dominance of pyrethroid resistance In the horn fly (Diptera Muscidae). 1 ECOQ Entomol. 79:1178-1182.
39. Subcommittee on Pesticides and Industrial Organic Chemicals. 1986. pyrethroids: Their effects on aquatic and terrestrial ecosystems. Publication No. NRCC 24376. Ottawa, Canada National Research Council of Canada; !
40. Saini, RK, N.P. Chopra, and A N. Verma. 1989. Development of insecticide resistance and cross-resistance In fenvalerate-, and cypermethrin-selected strains of Earias vittella`(F&b.). Pestic. Sci. 25:289-295.
41. Whalon, M. and R Hollingworth (eds.) 1990. Pest resistance management. Vol. 2 No. 1. Lansing, Ml: Pesticide Research Center.
42. Croft, B.A. et al. 1989. Local and regional resistance to fenvalerate In Psylla pyricola Foerster (Homoptera: Psyllidae) In western North America. Can. Entomol 121:121129.
Citation for this article: Mueller - Beilschmidt, Doria 1990, "Resistance of insect pests and disease vectors to synthetic pyrethroids", Journal of Pesticide Reform , Vol. 10, No. 4,Winter 1990 - 1991, pp. 34 -38.
Copyright © 1990 Northwest Coalition for Alternatives to Pesticides.
Reprinted with permission.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: info@eap.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster