AJAA Index | Virtual Library | Magazine Rack | Search
Abstract. Biological control of arthropod pests has a long history of useful practical application. Parasites, predators, and pathogens have been employed in many cases to control pest arthropods in an efficient, colt-effective, and permanent manner. The traditional tactics used in biological control (classical, augmentation, and conservation) remain vital and valuable tools in the biological control of pests for agricultural crops, range lands, forests, and glasshouses New technologies offer promise. One emerging technique involves the genetic improvement of natural enemies of arthropods through selection, hybridization, or recombinant DNA technology.
Key words: biocontrol agents, improvement, selection, hybridization, recombinant DNA technology, forests, rangelands, farmlands
Crop yields in the United States are reduced by the impact of a variety of pests. Weeds are potentially the most damaging, followed by approximately 8000 species of insects and at a longer lever by plant pathogens and nematodes (Baba, 1982). Control of pest insects has been achieved through chemical, cultural, and biorational controls, but biological control has unique advantages over the other tactics.
Biological control has been defined as the "actions of parasites, predators, and pathogens in maintaining another organism's density at a longer average than would occur in their absence" (DeBach, 1964). Some scientists would include host plant resistance, autocidal control, and pheromones under the category of biological control, but, while these biorational approaches to pest control have a biological basis, many investigators do not consider them to be biological control. Generally, biological control has been achieved by the use of one of three approaches--classical, augmentation, or conservation.
"Classical" biological control is based on the importation of exotic naturel enemies (parasites, predators, or pathogens) and their long-term establishment in the new environment, a strategy which may then provide long-term control of the target exotic pest arthropod or weed. This approach to biological control has been rewarding; hundreds of successful projects have reduced damage caused by a wide array of exotic pest arthropods and weeds (Laing and Hamai, 1976; Clausen, 1978). In addition, once a successful program is achieved in one location, the same naturel enemies are frequently used to control the same pests elsewhere in other climatically similar locations. Around the world, about 170 successes were achieved between 1964 and 1976 by introducing naturel enemies into a second site after they had been proven effective in the original geographic site of introduction (Luck et al.,1988). Natural enemy importation thus remains an important and effective tactic in pest management, particularly in the management of those arthropod and weed pests that are exotic. This approach to biological control, unfortunately, has received less support than it deserves.
While many scientists are worried about the extinction of animal and plant species in ecosystems, agricultural pest management specialists are concerned about additions to the fauna and flora of agroecosystems. In 1971, the U.S. Department of Agriculture established a task force to review the effectiveness of plant quarantines in preventing the entry of exotic pests and to quantify the risks associated with the entry of such pests and diseases (Sailer, 1983). A list of immigrant insects and related arthropods in the United States was compiled that categorized the immigrants as pests, beneficial species, and those of no known economic importance. The list continues to grow as additional species are found. At the conclusion of the 1971-1972 study, 1115 species were recognized to be of foreign origin and this number had increased to 1385 by 1977. Sixteen insect orders, mites (Acarina), and spiders (Araneae) are represented among the exotic arthropod species.
Foreign insect and mite species are considered to be responsible for a major part of all crop losses; one estimate is that they are responsible for 50 percent of such losses in California (Sailer, 1983). When viewed nationally, foreign species comprise 39 percent (235) of approximately 600 important arthropod pest species. Another 630 foreign species are on the list as pests of lesser importance, and an additional 420, or almost 25 percent of the immigrant fauna, are species of no known importance, while the remaining 398 are in some degree beneficial (Sailer, 1983). Exotic invaders include such pests as the Japanese beetle, European corn borer, Florida red scale, Rhodesgrass scale, spotted alfalfa aphid, gypsy moth, cottony cushion scale, California red scale, olive parlatoria scale, European red mite, imported southern red fire ant, Russian wheat aphid, and boll weevil.
Arthropods probably will continue to be added to the fauna of the United States at the rate of about eleven species per year, despite the efficacy of the national quarantine system (Sailer, 1983). Of the eleven, seven are likely to be pests of some importance, and about every third year a pest of major significance will be discovered. Assuming that many of these pests are not or cannot be eradicated, classical biological control will remain of crucial importance in controlling new exotic pests in forests, range lands, and agroecosystems.
Classical biological control has been actively practiced for about 100 years in the United States. Worldwide, approximately 2300 introductions directed against insect pests have provided complete biological control in about 100 cases (Ehler and Andres, 1983). Substantial control was provided in an additional 140 cases. Estimates of project outcomes that are successful range from 16 to 34 percent. Many factors affect success in classical biological control programs, including climate, natural enemies, habitat type, genetics, host compatibility, host phenology, and operational procedures. Thus, while classical biological control is effective and has yielded complete and lasting control in many important situations, there are several aspects of this pest management tactic that require additional research (Ehler and Andres, 1983; Hoy, 1985a). As noted by Huffaker (1971), "Many attempts have been only casual or have involved use of a particular natural enemy against unsuitable species or in unsuitable environments. The possibilities for controlling the notorious codling moth in this way, or the Mexican bean beetle, or the cotton boll weevil, for example, have only been explored superficially."
There are many views as to what the research priorities in classical biological control should be. There is little disagreement about the fact that this control has been under-exploited and underfunded. In one sense, it has been oversold; the dramatic cases in which complete biological control has been achieved through a small investment in research funds has, in my opinion, resulted in unrealistic expectations as to the resources needed to properly conduct classical biological control programs. This could be labeled the "cottony cushion syndrome."
The cottony cushion scale threatened the citrus industry in California in the late 1800s. This exotic species was suspected to have come from Australia. A very small amount of funding ($2000) was obtained to support a field agent's search for natural enemies in Australia in 1888. A parasite and a small lady beetle (then called Vedalia) were found attacking the scale in the Adelaide area of Australia, and 129 beetles were shipped to California. There they were placed on a small infested citrus tree enclosed in a mesh tent in January, 1889. By early April the scales were controlled on the caged tree and by early June over 10,000 beetles had been distributed to various southern California groves. By the end of 1889 the Vedalia beetle had cleared the scale pest from hundreds of acres of citrus. Unfortunately, most classical biological control programs probably require substantially more time and resources than were necessary for this project, yet such resources are rarely provided. However, if they were provided, both the rate of establishment and rate of success in classical biological control of arthropod pests and weeds could probably be increased, thus providing long-term, economical, and environmentally sound control of exotic pests (Huffaker, 1971; Tauber et al., 1985).
Augmentation involves efforts to increase populations or beneficial effects of natural enemies of both native and exotic pests (Rabb et al., 1976). A comprehensive review of augmentation in biological control is found in Ridgway and Vinson's (1977) book on Biological Control by Augmentation of Natural Enemies. Augmentation involves various techniques, including periodic releases and environmental manipulation. Periodic releases may be labeled inundative or inoculative, depending upon the numbers of natural enemies released and the interval during which they are expect' to provide control. Environmental manipulation may include provision of alternative, factitious hosts or prey, use of semiochemicals to improve natural enemy performance, provision of environmental requisites such as food or nesting sites, and modification of cropping practices to favor natural enemies. Augmentation has been particularly successful glasshouse crops (Hussey and Scopes 1985).
Inundative releases are designed to control a pest by the actions of the released natural enemies, not by the actions of their progeny, and thus can be considered "biotic insecticides." Inundative releases are currently hampered by our limited ability to produce high quality, inexpensive, mass-reared natural enemies. Thus, advances in current research on synthetic diets, artificial hosts, quality control, and genetic manipulation could result in increased of this tactic (King and Leper, 19 Hoy, 1986).
Another emerging technology is the use of semiochemicals to improve the efficacy of natural enemies in augmentation schemes. Parasitic insects use various chemical cues to locate their hosts (Nordlund et al., 1981). Recent reports, indicate that learning can modify the responses of parasites to these chemicals (Lewis and Tumlinson, 1988). The complexity of host seeking behavior exhibited by arthropod natural enemies is only beginning to be understood and could lead to the more sophisticated use of natural enemy augmentation.
Conservation involves protecting and maintaining natural enemy population Conservation is crucial if both native and exotic natural enemies are to be maintained in agricultural ecosystems. Most commonly, conservation involves modifying pesticide application practices so that they occur only when the pest population exceeds specified levers. In some cases, conservation of naturel enemies can be achieved by changing the active ingredient, rates, formulations, timing, and location of pesticide applications (Hull and Beers, 1985). Or, existing populations of naturel enemies can be protected by maintaining refuges. According to Tauber et al (1985), "It is probable that the most dramatic increase in the utilization of biological control in agricultural IPM systems could come through the judicious use of selective pesticides in conjunction with effective naturel enemies in specific cropping systems, in specific geographic regions. While we have some knowledge of pesticide selectivity, it is woefully inadequate to generally allow such precise usage." As long as key pests cannot be controlled biologically, culturally, or through host plant resistance, agricultural chemicals will be needed. Learning how to conserve naturel enemies in the agroecosystem is an effective way to increase the use of biological control in agriculture (Hull and Beers, 1985).
Genetic manipulation of naturel enemies of arthropods offers promise of enhancing their efficacy in agricultural cropping systems. Genetic manipulation of other beneficial arthropods, such as silkworms and honey bees, has been conducted for hundreds of years (Hoy, 1986). Such manipulation of biological control agents seems to be a logical extension of the domestication of crop plants and animals that has been part of agriculture for thousands of years, since many agricultural systems are artificial. As in crop breeding, three potential genetic manipulation tactics exist, i.e., artificial selection, hybridization (use of heterosis), and recombinant DNA (rDNA) techniques. To date, only artificial selection of arthropod naturel enemies has been successfully employed, and the potential role of heterosis or rDNA technologies remains to be documented (Hoy, 1985c, 1986; Beckendorf and Hoy, 1985).
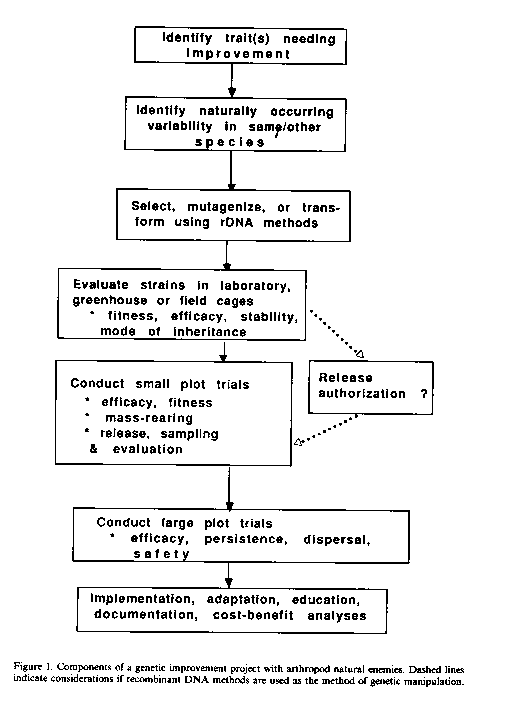
What are some of the constraints to initiating a genetic improvement project of arthropod naturel enemies? First, the factors limiting the efficacy of the natural enemy must be identified (Figure 1). This means that a great deal must be known about the biology, ecology, and behavior of the naturel enemy. This first step is extremely crucial, since improper identification of the trait needing improvement could lead to an expensive and time-consuming project of little practical value. Second, genetic variability must be available upon which one can select if using artificial selection. If such variability does not occur in naturel populations, it must be provided for through mutagenesis or, perhaps, through recombinant DNA methods. Third, the "improved" naturel enemy must be documented to be effective in the field. Finally, one must assume that the cost of the project will be justified by the benefits achieved.
Headley and Hoy (1986) recently reviewed the benefits and costs of an integrated mite management program in California almond orchards that involves the use of a genetically manipulated predatory mite. Metaseialus occidentalis (Nesbitt) is an effective predator of spider mites in deciduous orchards and vineyards in western North America (Hoy, 1985b). It acquired resistance to organophosphorus insecticides (OPs) through natural selection in apple orchards in Washington and this resistance allowed the predator to survive in orchards even though an OP insecticide (azinphosmethyl or Guthion) was applied to control codling moth (Hoyt, 1969). In 1977, a genetic improvement project with M. occidentalis was initiated, with the goal of developing additional pesticide resistance in this predator in order to increase its usefulness in orchard and vineyard pest management programs.
Selection for resistance to carbaryl and permethrin was successful, and multi-resistant strains of M. occidentalis were obtained through laboratory crosses and additional selections. The laboratory-selected strains were then tested in small plot trials for two years, to determine whether they could become established in orchards or vineyards, survive the relevant pesticide applications in the field, spread, multiply, overwinter, and control the spider mites (Hoy, 1985b). The small plot trials were then followed by three years of research to learn how to implement the predators in an integrated mite management program in almonds (Hoy, 1985b; Headley and Hoy, 1986). Implementation involved developing mass rearing methods, monitoring methods, and learning how to use reduced rates and numbers of applications of insecticides and acaricides selective to this predator. The economic analysis suggested that almond growers who adopted the program would save $60 to $110/hectare. The programatic benefit/cost analysis suggested that the return on the research investment will range from 280 to 370 percent per year, depending upon the level of adoption by almond growers on the 158,000 hectares of almonds grown in California. This high rate of return on research investment was attributed, in part, to the fact that more than half of the research resources were allocated to field testing and implementation research (Headley and Hoy, 1986). Thus, genetic improvement with M occidentalis has been shown to be efficacious and cost effective.
Genetic improvement projects with several phytoseiid species have included selection for enhanced fecundity, temperature tolerance, and non-diapause as well as pesticide resistance (Hoy,1985c, Table 1). Selection projects are currently being conducted in the U.S.A., China, New Zealand, and France and field trials are being conducted with some of the selected strains. The successful implementation and economic analyses of costs and benefits of these genetically manipulated predatory mites will provide impetus to this tactic in biological control.
Genetic improvement projects with natural enemies of insects have been conducted for improved climatic tolerances, improved host finding ability, changes in host preference, improved synchronization with the host, insecticide resistance, non-diapause, and induction of thelytokous reproduction (Table 2), but to date none of these genetically manipulated natural enemies has been used in the field. Several current projects also are in progress and planned field trials will determine whether use of genetically manipulated insect natural enemies can be implemented in agricultural ecosystems. Aphytis melinus. a parasite of the California red scale, has been selected for resistance to carbaryl and the resistance level achieved appears to be sufficiently high that field trials are justified to evaluate this strain's efficacy in California citrus orchards (Rosenheim and Hoy, 1988). The walnut aphid parasite Trioxys pallidus, has been selected for resistance to azinphosmethyl, and this strain was tested in California walnut orchards during the 1988 growing season (Hoy and Cave, 1988). If A. melinus and T. pallidus are able to establish, survive, parasitize their hosts, and successfully overwinter, then genetic improvement may be documented to be effective with insect parasitoids as well as with predatory mites.
The potential for using rDNA technology in genetic manipulation of natural enemies of arthropods has been reviewed by Beckendorf and Hoy (1985). This new technology offers the possibility that genetic manipulation could become more efficient and more creative; beneficial genes isolated from one species could be transferred to many if efficient transformation systems can be found. This research has a number steps; desirable genes must be identified cloned, inserted into natural enemies, be incorporated into their genome, be stable, be expressed in the appropriate t sues and at the appropriate time, and transmitted to the progeny. The transformed natural enemy strain must then be fit and able to perform well in agricultural systems (Figure 1). Issues relating to the safety of releasing rDNA manipulated arthropod naturel enemies remain to be resolved. Safety questions will probably focus on three issues: 1) whether the transformed strain is stable, 2) whether the improved strain retains its host/prey specificity, and 3) whether the ecological range of the transformed strain has been altered. It will be important to remember that genetic improvement of arthropod naturel enemies of insect and weed pests will be initiated because there is a serious pest problem. Risks and benefits of such genetic manipulation projects should be evaluated in terms of the costs and benefits of not doing the project.

In practice, effective biological control may require that several tactics be combined to achieve effective pest management. Thus, once an exotic naturel enemy is established, specific efforts may be needed to conserve it. Alternatively, it may have to be released augmentatively if it is unable to persist in sufficient numbers, perhaps because it lacks a diapause or alternative hosts. Classical, augmentation, and conservation tactics should not be considered to be mutually exclusive. Arthropods have been pests in agriculture for more than 10,000 years; it is likely that they will remain serious pests of agriculture, even as it changes and evolves. New technologies should be examined to determine whether they can result in improved biological control.
Biological control is not being fully exploited as a method for the control of pest arthropods in forests, range lands, and agriculture (Huffaker, 1971; Tauber et al., 1985). Additional and continuing efforts should be made in the traditional classical, augmentation, and conservation approaches to biological control because the benefits associated with these approaches are already known to be substantial and can probably be improved. In addition, research efforts should be continued to combine and develop new and innovative approaches to biological control, including the genetic manipulation of naturel enemies, the manipulation of naturel enemy behavior through semiochemicals, and the development of improved artificial diets and improved rearing methods so that mass production for augmentative releases becomes economical. As summarized by Tauber et al. (1985), research needs in biological control "...form a continuum from the very basic to the applied..."
1. Abdelrahman, 1. 1973. Toxicity of malathion to the naturel enemies of California red scale Aonidiella aurantii (Mask.) (Hemiptera:Diaspididae). Australian Journal of Agricultural Research 24:119-133.
2. Adams, C. H., and W. H. Cross. 1967. Insecticide resistance in Bracon mellitor, a parasite of the boll weevil. Journal of Economic Entomology 60:1016-1020.
3. Allen, H. W. 1954. Propagation of Horogenes molestae, an Asiatic parasite of the Oriental fruit moth, on the potato tuberworm. Journal of Economic Entomology 47:278-281.
4. Avella, M., D. Fournier, M. Pralavorio, and J. B. Berge. 1985. Selection pour la resistance a la deltamethrine d'une souche de Phytoseiulus persimilis Athias-Henriot. Agronomie 5:177-180.
5. Batra, S. W. T. 1982. Biological control in agroecosystems. Science 215:134-139.
6. Beckendorf, S. K., and M. A. Hoy. 1985. Genetic improvement of arthropod naturel enemies through selection, hybridization or genetic engineering techniques. In M. A. Hoy and D. C. Herzog (eds.). Biological Control in Agricultural IPM Systems, Academic Press, Orlando, Florida. pp. 167-187.
7. Box, H. E. 1956. Battle against Venezuela's cane borer. I. Preliminary investigations and the launching of a general campaign. Sugar, 25-27:30,45.
8. Clausen, C. P., editor. 1978. Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review. Agricultural Handbook 480, USDA-ARS, Washington, DC.
9. DeBach, P., editor. 1964. Biological Control of Insect Pests and Weeds. Chapman and Hall, London, England. 844 pp.
10. Ehler, L.E., and L. A. Andres. 1983. Biological control: exotic naturel enemies to control exotic pests. In C. L. Wilson and C. L. Graham (eds.). Exotic Plant Pests and North American Agriculture, Academic Press, New York, New York. pp. 395-418.
11. Field, R. P., and M. A. Hoy. 1986. Evaluation of genetically improved strains of Metaseialus occidentalis (Nesbitt) (Acarina: Phytoseiidae) for integrated control of spider mites on roses in greenhouses. Hilgardia 54(2):1-32.
12. Gilkeson, L. A., and S. B. Hill. 1986. Genetic selection for and evaluation of non-diapause fines of predatory midge, Aphidoletes aphidimyza (Rondani) (Diptera: Cecidomyiidae). Canadian Entomologist 118:869-879.
13. Graflon-Cardwell, E. E., and M. A. Hoy. 1986. Genetic improvement of common green lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae): selection for carbaryl resistance. Environmental Entomology 15:11301136.
14. Hagen, K. S., and J. M. Franz. 1973. A history of biological control. In R. F. Smith, T. E. Mittler, and C. N. Smith (eds.). History of Entomology. Annual Reviews, Inc., Palo Alto, California. pp. 433-476.
15. Headley, J. C., and M. A. Hoy. 1986. Benefit/ cost analysis of an integrated mite management program for almonds. J. Econ. Entomol. 80:555-559.
16. Hoy, M. A. 1984. Genetic improvement of a biological control agent: multiple pesticide resistance and nondiapause in Metaseialus occidentalis (Nesbitt) (Pytoseiidae). Proceedings VI International Congress of Acarology, Edinburgh, 1982, Acarology VI, Volume 2. D. A. Griffiths and C. E. Bowman, editors, Ellis Horwood Ltd., Halsted Press, New York New York. pp. 673-679.
17. Hoy, M. A. 1985a. Improving establishment of arthropod naturel enemies. In M.A. Hoy and D. C. Herzog (eds.). Biological Control in Agricultural IPM Systems. Academic Press, Orlando, Florida. pp. 151 - 166.
18. Hoy, M. A. 1985b. Integrated mite management for California almond orchards. In W. Helle and M. W. Sabelis (eds.). Spider Mites, Their Biology, Natural Enemies and Control. Volume IB. Elsevier Science Publ., Amsterdam. pp. 299-310.
19. Hoy, M. A. 1985c. Recent advances in genetics and genetic improvement of the Phytoseiidae. Annual Review of Entomology 30:345-370.
20. Hoy, M. A. 1986. Use of genetic improvement in biological control. Agriculture, Ecosystems, and Environment 15: 109-119.
21. Hoy, M. A., and F. E. Cave. 1988. Selection of the walnut aphid parasite, Trioxys palliadus, for resistance to azinphosmethyl. California Agriculture 42(4):4-5.
22. Hoy, M. A., and N. F. Knop. 1981. Selection for and genetic analysis of permethrin resistance in Metaseialus occidentalis: genetic improvement of a biological control agent. Entomologia Experimentalis et Applicata 30:10-18.
23. Hoyt, S. C. 1969. Integrated chemical control of insects and biological control of spider mites on apple in Washington. Journal of Economic Entomology 62:74-86.
24. Huang, M.-D., J.-J. Xiong, and T.-Y. Du. 1987. The selection for and genetical analysis of phosmet resistance in Amb/yŠius nicholsi. Acta Entomologica Sinica 30(2):133-139.
25. Huffaker, C. B., editor. 1971. Biological Control. Plenum Press, New York, New York. 511 pp.
26. Hull, L. A., and E. H. Beers. 1985. Ecological selectivity: modifying chemical control practices to preserve naturel enemies. In M. A. Hoy and D. C. Herzog (eds.). Biological Control in Agricultural IPM Systems. Academic Press, Orlando Florida. pp. 103-122.
27. Hussey, N. W. and N. Scopes, editors. 1985. Biological Pest Control, The Glasshouse Experience. Cornell University Press, Ithaca, New York. 240 pp.
28. King, E. G., and N. C. Leppla, editors. 1984. Advances and Challenges in Insect Rearing. USDA, ARS (Southern Region), New Orleans, Louisiana. 305 pp.
29. Laing, J. E., and J. Hamai. 1976. Biological control of insect pests and weeks by imported parasites, predators and pathogens. In C. B. Huffaker and P. S. Messenger (eds.). Theory and Practice of Biological Control. Academic Press, New York, New York. pp. 685-743.
30. Landaluz, P. U. 1950. Aplicacion de la genetica al aumento de la eficacia del Trichogramma minutum en la lucha biologica. Bolet. Patol. Vegetal Entomol. Agric. 18:1-12.
31. Legner, E. F. 1987. Transfer of thelytoky to arrhenotokous Muscidifurax raptor Girault and Sanders (Hymenoptera: Pteromalidae). Canadian Entomologist 119:265-271.
32. Lewis, W. J., and J. H. Tumlinson. 1988. Host detection by chemically mediated associative learning in a parasitic wasp. Nature 331:257259.
33. Luck, R. F., B. M. Shepard, and P. E. Kenmore. 1988. Experimental methods for evaluating arthropod naturel enemies. Annual Review of Entomology 33:367-391.
34. Markwick, N. P. ;986. Detecting variability and selecting for pesticide resistance in two species of phytoseiid mites. Entomophaga 31 :225-236.
35. Nordlund, D. A., R. L. Jones, and W. J. Lewis, editors. 1981. Semiochemicals, Their Role in Pest Control. W. J. Wiley, Chichester.
36. Pielou, D. P., and F. R. Glasser. 1952. Selection for DDT resistance in a beneficial insect parasite. Science 115:117.
37. Rabb, R. L., R. E. Stinner, and R. van den Bosch. 1976. Conservation and augmentation of naturel enemies. In C. B. Huffaker and P. S. Messenger (eds.). Theory and Practice of Biological Control. Academic Press, New York, New York. pp. 233-254.
38. Ram, A., and A. K. Sharma. 1977. Selective breeding for improving the fecundity and sex J ratio of Trichogramma fasciatum (Perkins) (Trichogrammatidae: Hymenoptera), an egg parasite of lepidopterous hosts. Entomology 2:133-137.
39. Ridgway, R. L., and S. B. Vinson 1977. Biological Control by Augmentation of Natural Enemies. Plenum Press, New York, New York. 480 pp.
40. Robertson, J. G. 1957. Changes in resistance to DDT in Macrocentrus ancylivorus Rohw. (Hymenoptera: Braconidae). Canadian Journal of Zoology 35:629-633.
41. Rosenheim, J. A., and M. A. Hoy. 1988. Genetic improvement of a parasitoid biological control agent: artificial selection for insecticide resistance in Aphytis melinus (Hymenoptera: Aphelinidae). Journal of Economic Entomology.
42. Roush, R. T., and M. A. Hoy. 1981. Genetic improvement of Metaseialus occidentalis: selection with methomyl, dimethoate, and carbaryl and genetic analysis of carbaryl resistance. Journal of Economic Entomology 74:138-141.
43. Sailer, R. I. 1983. History of insect introductions. In C. L. Wilson and C. L. Graham (eds.). Exotic Plant Pests and North American Agriculture. Academic Press, New York, New York. pp. 15-37.
44. Schulten, G. G. M., and G. van de Klashorst. 1974. Genetics of resistance to parathion and demeton-s-methyl in Phytoseiulus persimilis. A. H. (Acari: Phytoseiidae). Proceedings 4th International Congress of Acarology. pp. 519524.
45. Simmonds, F. J. 1947. Improvement of the sex-ratio of a parasite by selection. Canadian Entomologist 79:41-44.
46. Strickler, K. A., and B. A. Croft. 1982. Selection for permethrin resistance in the predatory mite Amblyseius fallacis Garman (Acarina: Phytoseiidae). Entomologia Experimentalis et Applicata 31:339-345.
47. Szmidt, A. 1972. Studies on the efficiency of various strains of the parasite Dahlbominus fuscipennis (Zett.) (Hymenoptera, Chalcidoidea) under naturel condition. Ekol. Pol. 20:299-313.
48. Tauber, M. J., M. A. Hoy, and D. C. Herzog. 1985. Biological control in agricultural IPM systems: a brief overview of the current statue and future prospects. In M. A. Hoy and D. C. Herzog (eds.). Biological Control in Agricultural IPM Systems. Academic Press, Orlando, Florida. pp. 3-9.
49. Urquijo, P. 1946. Selecion des extirpes de Trichogramma minutum Riley de maxima effectividad parasitaria. Boln. Patol. Veg. Ent. Agric. 14:199-216.
50 Voroshilov, H. V. 1979. Heat-resistant fines of the mite Phytoselulus persimilis H.-H. Genetika 15(1):70-76.
51. Voroshilov, N. V., and L. I. Kolmakova.1977. Heritability of fertility in a hybrid population of Phytosefulus. Genetika 13(8):1496-1497.
52. Weseloh R. M. 1986. Artificial selection for host suitability and developmental length of the gypsy moth (Lepidoptera: Lymantriid parasite, Cotesia melanoscela (Hymenoptera Braconidae). J. Econ. Entomol. 79:1212-12
53. White, E. B., P. DeBach, and M. J. Garber 1970. Artificial selection for genetic adaptation to temperature extremes in Aphytis lingnanensis Compere (Hymenoptera Aphelinidae). Hilgardia 40(6):161-192.
54. Wilkes, A. 1942. The influence of selection on the preferendum of a chalcid (Microplectron fuscipennis Zett.) and its significance in biological control of an insect pest. Proc. Royal Society London. Series B 130:400-t
Citation : Hoy, Marjorie A., 1988, " Biological control of arthropod pests : traditional and emerging technologies", Vol. 3, No. 2 and 3, pp. 63-68.
Copyright ę 1988
Reprinted with permission.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: info@eap.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster