

EAP Publications | Virtual Library | Magazine Rack | Search | What's new
Join the Ecological Solutions Roundtable
This underutilised plant has promising economic value. The challenge is to find ways to incorporate it into existing food products, as well as to create new products from it
Rita A. Teutonico and Dietrich Knorr
THE ROLE OF AMARANTH as an under exploited plant with promising economic value has recently been recognized by the National Academy of Sciences (NAS, 1975; 1984). The Amaranthaceae family consists of hardy, weedy, herbaceous, fast-growing, cereal-like plants (Opute, 1979), with a seed yield of up to 3 tons/ hectare when grown in monoculture for 3-4 months, and a vegetable yield of 4.5 tons dry matter/hectare after 4 weeks (Grubber and van Sloten, 1981). Amaranth is one of those rare plants whose leaves are eaten as a vegetable while the seeds are used as cereals (Oke, 1983; Saunders and Becker, 1984; Kauffman and Haas, 1983). The principal species of Amaranthus and their synonymous names, origins, and uses are listed in Table 1.
Several amaranth species have been cultivated in the Old and New World since ancient times as grain crops, pot herbs, ornamentals, and dye plants (Saver, 1950a). The potential of both grain and vegetable amaranth as a food resource has been reviewed extensively by Haas and Kauffman (1984), Saunders and Becker (1984), NAS (1984), and Sanchez-Marroquin (1980). Dye use seems limited to cultures who do not grow amaranth as a grain crop (Sauer, 1950a). The red dye from amaranth leaves is used to colour alcoholic beverages in Bolivia and northwestern Argentina, to colour maize dough in Mexico and the southwestern United States (Sauer, 1950a), and to dye foods and beverages in Ecuador (Jain and Hauptli, 1980).
This article reviews the history, composition, and present and potential food uses of grain and vegetable amaranth.
The earliest archaeological record of pale-seeded grain amaranth is that of A. cruentus, found in Tehuacan Puebla, Mexico, about 4000 BC (Pal and Khoshoo, 1974; Sauer, 1979), making it one of the oldest known food crops; it probably originated in Central and South America (Grubber and van Sloten, 1981). Amaranth was a major grain crop in the pre-conquest Aztec empire (Sauer, 1950b; Pal and Khoshoo, 1974; Early, 1977; Haughton, 1978); ancient Mexicans made idols of a dough from seeds of the crop they called huahtli, which has been identified as grain amaranth (Sauer, 1950b; Marx, 1977).
Pale-seeded amaranths were also grown in Germany in the 16th century, India and Ceylon in the 18th century, the Himalayas in the early 19th century, and interior China and Eastern Siberia in the late 19th century (Sauer, 1977).
Present Uses. A. caudatus, A. cruentus, and A. hypochondriacus have been identified (NAS, 1975) as having the potential to increase world food production. A. caudatus is grown in the InterAndean Valleys of Peru and Bolivia (Sumar, 1983), A. cruentus is cultivated as a grain crop in Guatemala, and A. hypochondriacus is grown in Mexico (NAS, 1975). In Mexico, grain amaranth is used chiefly for making alegria candies from popped seeds and molasses (Early, 1977) and for preparing atole, a drink from roasted and powdered seeds mixed with syrup and water (Oke, 1983). In Peru, seeds are popped and ground into flour or bound with syrup and made into belles (Sumar, 1983). In India, the seeds are most commonly used in the form of candy known as laddoos (Vietmeyer, 1978), though the seeds are sometimes boiled with rice (Oke, 1983). Amaranth seeds are parched, ground into flour, and eaten as gruel (sattoo) in Nepal, while like chapattis in the Himalayas (Vietmeyer, 1978).
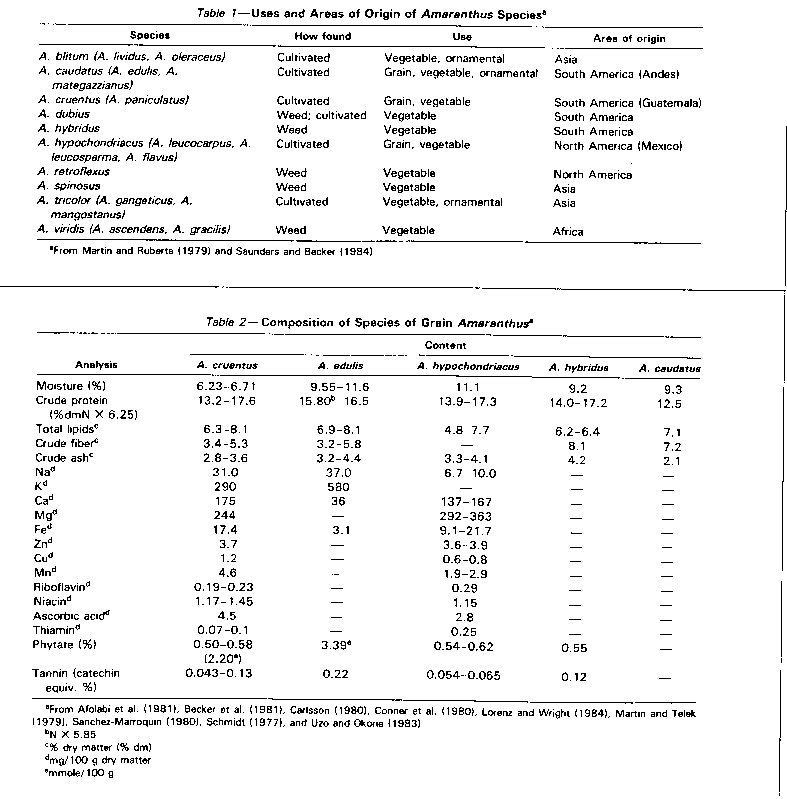
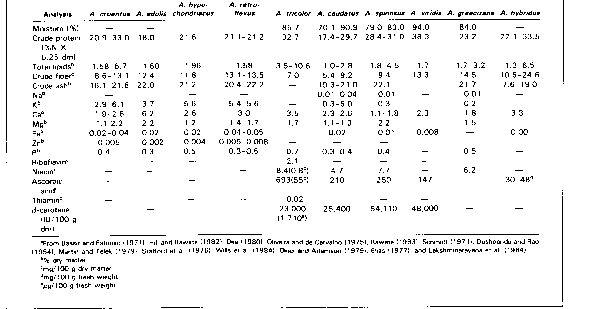
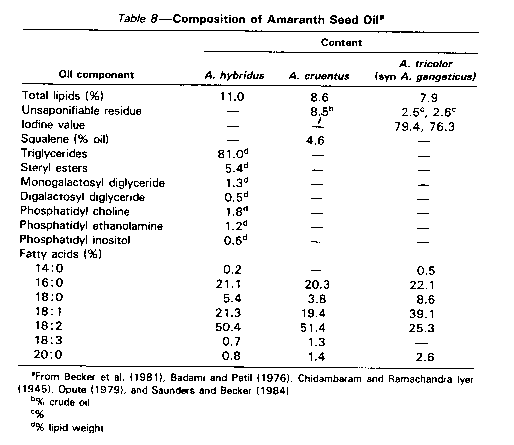
Composition. The crude protein content of grain amaranth (Table 2) ranges from 12.5 to 17.6 % dry matter. This is higher than in most common grains except soybeans. The mineral and vitamin composition and the phytate and tannin concentration of grain amaranth are also presented in Table 2. Grain amaranth protein contains around 5% lysine and 4.4% sulfur amino acids, which are the limiting amino acids in other grains (Senft, 1980). The amino acid composition of amaranth protein compares well with the FAD/WHO protein standard (Table 3). The total lipid content of grain amaranth ranges from 5.4 to 17.0% dry matter and has a high level of unsaturation (about 75%), containing almost 50 % linoleic acid (Opute, 1979; Carlsson, 1980; Becker et al., 1981; Badami and Patil, 1976).
· Seeds. Amaranth seeds are small and lenticular in shape, with each seed averaging 1.0-1.5 mm in diameter and 1,000 seeds weighing 0.6-1.2 g (Jain and Hauptli, 1980; Saunders and Becker, 1984). Amaranth grown for grain is pale-seeded, with seed colours ranging from off-white to brown (Irving et al., 1981; NAS, 1975; Saunders and Becker, 1984). Betschart et al. (1981) fractionated A. cruentus seeds by passing from five successive times through a modified Strong-Scott barley pearler and removed 25.5 cumulative percent of the seed-coat embryo. Nutrients were concentrated in the seed-coat embryo fraction, reaching 2.3-2.6 times as much nitrogen, fat, fibre, and ash, 2.4-3.0 times as much thiamin, riboflavin, and niacin, and 1.4 - 2.5 times as much of several mineral elements as the original, intact seed. Using a Brabender mill, Sanchez-Marroquin (1980) separated the seeds of A. hypochondriacus into coarse (16.2%) and fine (10.4%) flour fractions, plus 20.1% broken grains, and 52.6% "bran."
· Starch. The starch content of pale-seeded grain types was reported to range from 48% for A. cruentus to about 62 % for A. hypochondriacus (Becker et al., 1981; Saunders and Becker, 1984). The granules of starch isolated from the seeds of A. hypochondriacus were found to be small (1-3,um in diameter) and angular and polygonal in shape (Lorenz, 1981; Saunders and Becker, 1984; Stone and Lorenz, 1984), while those of A. cruentus were reported to be spherical as well as angular and polygonal (McMasters et al., 1955; Stone and Lorenz, 1984). According to Goering (1967), A. retropexus starch is composed of a small amount of small spherical granules and a large amount of irregular starch chunks. Physicochemical properties of chunk A. retropexus starch suggest a homogeneous mass very strongly bound together but very susceptible to the attack of amylases (Goering, 1967).
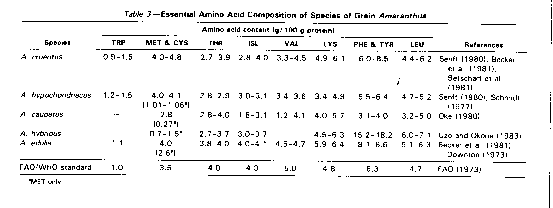
The existence of both nonglutinous and glutinous starches has been identified in A. hypochondriacus (Tomita et al., 1981; Okuno and Sakaguchi, 1981), with starch granules consisting of nearly 100% typical amylopectin. Sugimoto et al. (1981) reported that starch granules of two types of A. hypochondriacus contained 0 and 14% amylose, while Becker et al. (1981) found 7.2% amylose. X-ray diffraction analysis of A. hypochondriacus starches showed that they were identical to maize and rice starches, indicating A type crystalline structure (Sugimoto et al., 1981). The A. caudatus starch was reported to be completely nonglutinous (Okuno and Sakaguchi, 1981; 1982), while A. cruentus starch was reported to be glutinous (McMasters et al., 1955). Some properties of A. hypochondriacus and A. retropexus starches are given in Table 4.
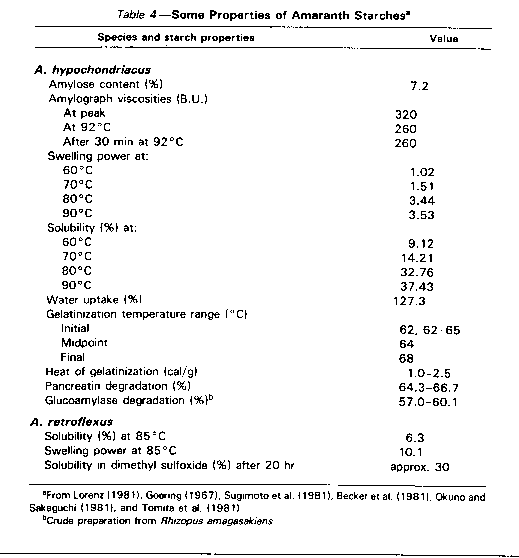
Lorenz (1981) reported that, compared to wheat starch, the starch of A. hypochondriacus has much a lower amylose content, a lower swelling power, a higher solubility, a greater water uptake, a lower amylograph viscosity, and a higher gelatinization temperature range. Becker et al. (1981) suggested that the very small size of the starch granules and residual amylase activity were presumably responsible for the observed differences in swelling power and solubility. The higher viscosity of wheat starch after cooling to 35°C is due to the higher amylose content's causing the development of aggregated structures with increased viscosity. Compared to corn starch, A. cruentus and A. hypochondriacus starches had higher swelling power, lower solubility, greater water uptake, lower susceptibility to a-amylase, higher amylograph viscosity, and much lower amylose content (Stone and Lorenz, 1984). High susceptibility of A. hypochrondriacus and A. caudatas starch granules to amylases was reported by Tomita et al. (1981).
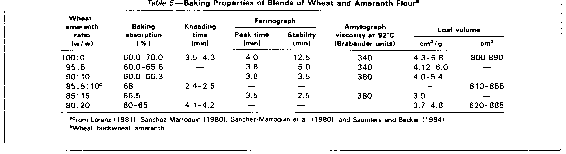
Flour and Baking Properties. The baking properties of amaranth seed flour and blends of wheat flour with up to 20% amaranth flour are presented in Table 5. Farinograph peak time and stabilities and specific loaf volume decreased with increasing A. hypochondriacus flour levels in the blend. Farinograph data (Lorenz, 1981) indicated a need for higher absorptions and shorter mixing times when using A. hypochondriacus flour as part of a composite flour in bread baking. According to Lorenz (1981), the flavour of the breads with amaranth was very pleasant and was preferred by a taste panel over the flavour of white bread. At substitution levels of 10 and 15%, the grain of the bread was more open, the texture not as silky, and the crumb colour slightly darker. Flour made from A. cruentus perisperm resulted in bread with the best combination of higher specific loaf volume and total score (Saunders and Becker, 1984). Breads and cakes baked with waxy-type amaranth starches were of poor quality (Stone and Lorenz, 1984). Crackers prepared with tezopaco wheat and 10, 20, and 30 % amaranth flour (Sanchez-Marroquin, 1980) resulted in comparable quality to the wheat cracker at amaranth levels up to 20%. The use of amaranth flour alone resulted in poor product texture. Because of its nutritional quality, amaranth flour has been successfully utilized as a supplement to corn flour in tortillas (Sanchez-Marroquin, 1980).
Effects of Processing. Heat treatment (toasting) has been recommended by Oke (1983) to overcome milling problems caused by the small size and grittiness of the seeds (Betschart et al., 1981). Popping of amaranth seeds resulted in an increase in volume of up to 1,050% (Saunders and Becker, 1984; Erwin, 1934). Betschart et al. (1981) reported that hot-air popping of A. cruentus seeds at an air temperature of 220 + 5°C for 10-15 see resulted in a protein efficiency ratio (PER) of 1.7 and an apparent nitrogen digestibility of 77%; these were not significantly different from those of the control seeds. Exposing whole seeds to 60°C had no effect on amino acid composition, ahead leucine, the first limiting amino acid, was not influenced by hot-air popping.
Tovar and Carpenter (1980) reported that popping amaranth resulted in a decrease in reactive lysine. Sanchez-Marroquin (1980) found improved PER levels in tortillas when popped amaranth was used for the corn-amaranth flour blend. Comparison of baking absorption and specific loaf volumes of breads prepared from blends of wheat flour with up to 20% A. cruentus flour resulted in slightly lower baking absorption. Heating the seeds to 60°C for 24 hr reduced the specific loaf volume compared to that of the control (Saunders and Becker, 1984). Germination of the seeds resulted in an increase in the concentration of some amino acids, particularly lysine (Sanchez-Marroquin et al., 1980).
Vegetable Amaranth
The main vegetable type of amaranth, A. tricolor , seems to have originated in South or Southeast Asia (Grubber and van Sloten, 1981) and then spread through the tropics and the temperate zone (Martin and Telek, 1979).
Present Uses. Many species of amaranth are grown as vegetables throughout the tropics and Eastern Asia (Feine et al., 1979), though only A. tricolor has been extensively cultivated, primarily in Southern China (Martin and Ruberte, 1979). A. cruentus is used as an African leafy vegetable but is actually a grain amaranth which was probably introduced from Central America (Grubber and van Sloten, 1981). It is also a popular pot herb (Martin and Telek, 1979), while A. caudatus, A. gracilis, A. graecizans, and A. spinosus are native foods in Mozambique (Oliveira and de Carvalho, 1975). The leaves and the softest portions of the shoots are usually boiled in several changes of water and then separated from the cooking liquid (Martin and Telek, 1979), though they traditionally are steamed in Uganda (Stafford et al., 1976). Amaranth leaves are combined with condiments to prepare soup in Nigeria (Oke, 1983; Okiei and Adamson, 1979); used in salad, boiled and mixed with a groundnut sauce in Mozambique (Oliveira and de Carvalho, 1975); or pureed into a sauce and served over (farinaceous) vegetables in West Africa (Martin and Telek, 1979). The flavor of raw and cooked vegetable amaranth was reported as equal to or better than that of spinach or other similar greens (Abbott and Campbell, 1982; Daloz, 1980; Martin and Ruberte, 1977).
· Composition. The harvested amaranth plant is 50-80 % edible (Oke, 1980), which only 20-30% of most vegetable plants is utilized directly for human consumption in the United States (Kramer and Kwee, 1977). Amaranth leaves contain 17.4-38.3 % dry matter as crude protein (Table 6), averaging 5% lysine and thus having potential as a protein supplement (Oliveira and de Carvalho, 1975). However, Cheeke et al. (1981) argued that the presence of saponins, alkaloids, phenolics, and oxalates might have a negative effect on leaf protein concentrate quality.
The major unsaturated fatty acids in A. tricolor are linoleic in seeds (49%) and stems (46%) and linolenic in leaves (42%), while the major saturated fatty acid in seeds, stems, and leaves is palmitic acid at 18-25% of total fatty acids (Fernando and Bean, 1984).
Vitamins C and A are present at nutritionally significant levels (Table 6), averaging 420 ppm of vitamin C and 250 ppm of §-carotene (Wills et al., 1984). Trace quantities of vitamin B-12-like activity were found in A. hypochondriacus leaves, though the exact nature of this activity could not be concluded (Jathar et al., 1974). Minerals such as potassium, iron, magnesium, and calcium (Table 6) exist also in significant concentrations, with average values of 287 ppm of iron and 2.1 % calcium (dry matter). The presence of large amounts of oxalate(s), ranging from 0.2 to 11.4% (dry weight), may limit availability of these nutrients.
Leaves. Probably the leaves of all 52-60 species of Amaranthus are ed~ble (Martin and Ruberte, 1979), with even the grain types, when young, being acceptable as vegetables (Mathai, 1978). Vegetable amaranth, such as Tete (A. chlorostachys) or Chinese spinach (A. tr~color), ~s commonly heat processed (Keshinro and Ketiku, 1979; Wills et al., 1984); typical processes include cooking, steaming, blanching, stir-frying, and baking (Oke, 1983; Saunders and Becker, 1984; Ajayi and Osibanjo, 1980; Stafford et al., 1976; Fafunso and Bassir, 1976).
Appearance, texture, flavor, and overall eating quality of 20 steamed Amaranthus species were compared to those of steamed spinach samples by a consumer sensory panel (Abbott and Campbell, 1982). A. dubius and most of the A. tricolor species did not differ significantly from spinach (P < 0.05) in all the sensory criteria examined, while A. cruentus was consistently considered less favorably (P < 0.05) than spinach. According to Der Marderosian et al. (1980), the oxalate and nitrate concentrations of vegetable amaranth are similar to those found in other leafy garden vegetables, and their presence does not significantly detract from the nutritional quality of amaranth greens. However, Wills et al. (1984) indicated that much of the calcium in A. tricolor could be in the form of calcium oxalate and hence biologically unavailable.
Leaf Protein Concentrate. Protein concentrates and isolates have been prepared from ground amaranth samples by expression of the juice, followed by pH adjustment, heat coagulation at 70-85°C, and subsequent dewatering (Cheeke et al., 1981; Carlsson, 1983; Hill and Rawate, 1982; Rawate, 1983).
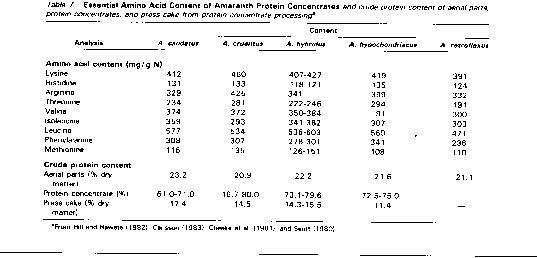
The protein content of aerial parts, protein concentrates, and press cakes, and the essential amino acid concentration of protein concentrates of various amaranth species are given in Table 7. Although the protein content of the concentrates from some varieties was comparable to those usually found in traditional protein concentrates (Rawate, 1983), the high ash content (up to 28% dry matter) and low crude protein content of some samples (Cheeke et al., 1981) may be attributable to the protein coagulation conditions used. Saunders and Becker (1984) reported that the lowest chemical score for amaranth leaf protein concentrates from few week-old plants was 95 and most of the essential amino acid scores were above 100, and that young amaranth leaves thus represent a valuable protein resource.
Fewer phenolics were found in plant concentrates than in the original plant matter (Cheeke et al., 1981). Hill and Rawate (1982) showed that press cakes obtained during amaranth leaf protein concentrate processing contained negligible amounts of nitrate and oxalate and would be a valuable feed source for ruminants.
At this time, we are not aware of any research work on functional properties of amaranth leaf protein concentrates.
Effects of Processing. Most of the work on the effects of processing on vegetable amaranth deals with the effects of various preparation methods on the vitamin C content of Tete (A. hybridus), which is widely consumed in Central Africa and parts of South Africa (Fafunso and Bassir, 1976). Keshinro and Ketiku (1979) reported an 80.3% loss of ascorbic acid from parboiling for 5 mini this increased to 91.5% after final cooking for 5 min. Cooking of fresh A. hybridus leaves resulted in a 35% loss of ascorbate and a reduction of the chemical score from 71 to 58 (Fafunso and Bassir, 1976). Blanching of 1-g (fresh-weight) portions of A. hybridus for 5 min in 10 ml of distilled water resulted in a reduction of the total vitamin C content from 560 to 228 mg/100 g dry matter (Ajay and Osibanjo, 1980). When boiling of whole leaves and boiling of finely chopped leaves were compared to steaming of leaves, steaming produced the least loss of nutrients such as ascorbic acid, iron, and protein, and also of oxalaté (Stafford et al., 1976).
Areas for Further Research
Among the areas in which further research is needed are the following:
Plant Improvement. The high genetic diversity in the amaranth family offers opportunities for increasing desirable characteristics, such as yield, protein content, and height (McKell, 1983). The fact that most of the seed volume is occupied by the embryo might account for the unusually high Iysine content (Oke, 1983); this provides a good opportunity for the development of varieties of even higher nutritional quality. Selection of plant varieties with large and nonshattering seeds, as well as the development of harvesting and processing methods adapted to the seed's characteristics (McKell, 1983), would improve processing of grain amaranth. Selection of vegetable amaranth low in nutritional stress factors, such as oxalate and nitrate, should improve nutritional quality and increase consumption of amaranth.
Finally, as Saunders and Becker (1984) argue, although there are claims that amaranths can flourish in stressful environments where conventional crops cannot, little evidence exists in the literature to document that amaranths are drought- or salt-tolerant. Plant tissue cultures of amaranth have been established (Ellis, 1973; Floresand Galston, 1980; Teutonico and Knorr, 1984a; b; c); these will provide in-vitro methods for crop improvement at the cellular level (Flores et al., 1982).
· Grain Amaranth Products. Currently, flour processed from amaranth seeds is being increasingly used in tortillas, breads, cookies, pasta, and marzipan (Sanchez-Marroquin et al., 1980) and has recently become available as an ingredient in a commercial breakfast cereal (Teutonico and Knorr, 1984a). Processing by extrusion cooking is being examined by Mexican researchers-(Del Valle, 1984). Sufficient data need to be collected on shelf-life characteristics, changes in nutritional quality during processing, and functionality of products.
Uses for amaranth seed oil which contains mainly nonpolar lipids, especially triglycerides, with a high degree of unsaturation (Table 8)still need to be identified and oil-refining processes established. The development of uses for amaranth seed coats and the processing of amaranth seed protein concentrates, and the nutritional implications of both are additional options for research and development aimed at commercialization of grain amaranth.
Vegetable Amaranth Plant Tissue Culture. Attempts have been initiated to reduce nutritional stress factors, such as oxalate and nitrate, of vegetable amaranth via plant tissue culture methods and to examine cell growth (Teutonico and Knorr, 1984a; b). Reduction of oxalate and nitrate in cultured amàranth cells could lead to direct use of the plant biomass produced by cell culture as a food product where low levels of nutritional stress factors, sterility, small particle size, and soft texture are product requirements (e.g., baby food or geriatric diets). Regeneration of such cells into plants could also result in new amaranth varieties with reduced oxalate content, thus improving overall productivity (nutrient availability) of the plants.
Suspension cultures of A. tricolor with molar ratios of nitrate and ammonium in the medium ranging from 12.5:1.0 to 1.0:12.5 were used to examine the effect of these nitrogen sources on growth and on oxalate and nitrate concentrations (Teutonico and Knorr, 1984b; c). Fresh-weight growth indices ranged from 0.6 + 0.06 to 4.6 + 0.24, indicating a significant effect of media selection on growth of cultured cells. Oxalate and nitrate concentrations of suspended A. tricolor cells were significantly (P < 0.01) lower than those of seedlings grown on the same medium, with the overall means of nitrate and oxalate concentration being reduced from 40.5 + 15.8 to 3.2 + 3.9 mg/g dry matter and 41.4 + 20.8 to 6.8 + 7.8 mg/g dry matter, respectively. A nondestructive method was developed (Teutonico and Knorr, 1985) for screening cultured cells of A tricolor for the isolation of low oxalate variants which upon regeneration could produce plants with reduced oxalate content.
Pigment Production. Mature leaves of A. tricolor and A. caudatus contain red-violet pigments-- the betacyanins amaranthin and isoamaranthin (Piatelli et al., 1969). They are derivatives of betanidin, which is formed from 3,4-dihydroxyphenylalanine (Stobart et al., 1970). Betacyanin formation in Amaranthus cotyledons is a light controlled process, but it can also be induced by the plant hormones, cytokinins, and their analogs (Obrenovic, 1983). Preliminary experiments in our laboratory indicate the potential of cultured A. tricolor cells for production of betacyanins.
A Challenge
The aforementioned composition, properties, and historical, current, and future applications of amaranth demonstrate the food potential of this underutilized crop. Nevertheless, there are problems in commercialization of amaranth, mainly because of lack of sufficient experimental data.
In the area of agriculture, the specific soil nutrient requirements of the amaranth plant, the effects of fertilization on its yield, and the plant composition at different stages of harvest still need to be explored in more detail. Also, selection is needed for those varieties that are most stress-tolerant and most productive in a temperate climate.
In the food processing area, research and development work is needed on shelf life, the functionality of grain amaranth and amaranth protein concentrates, and the effects of processing on functionality and nutritional quality of amaranth leaves and seeds. In addition, the small size of the amaranth seed has been considered a deterrent to commercialization, so either varieties must be bred for larger seeds or processing methods must be adapted to the small grain. The main challenge for R&D is to incorporate amaranth into existing food formulations to modify their functional and nutritional quality, as well as to create entirely new products from grain and vegetable amaranth.
References
Abbott, J.A. and Campbell, T.A. 1982. Sensory evaluation of vegetable amaranth (Amaranthus spp.). Hort Science 17: 409.
Afolabi, A.O., Oke, O.L., and Umoh I.B. 1981. Preliminary studies on the nutritive value of some cereal-like grains. Nutr. Rept. Intl. 24: 389.
Ajayi, S.O. and Osibanjo, O. 1980. Vitamin C losses in cooked fresh leafy vegetables. Food Chem. 5: 243.
Badami, R.C. and Patil, K.B. 1976. Studies on vegetable oils of genus amaranthus. J. Oil Technol. Assoc. India 8: 131.
Bassir, O. and Fafunso, M. 1971. Variations in the protein, carbohydrate, lipid and ash content of six tropical vegetables. Plant Foods for Man 1: 209.
Becker, R., Wheeler, E.L., Lorenz, K., Stafford, A.E., Grosjean, O.K., Betschart, A.A. and Saunders, R.M. 1981. A compositional study of amaranth grain. J. Food Sci. 46: 1175.
Betschart, A.A., Irving, D.W., Shepherd A.D., and Saunders, R.M. 1981. Amaranthus cruentus: Milling characteristics distribution of nutrient within seed components, and the effects of temperature on nutritional quality. J. Food Sci. 46: 1181.
Carlsson, R. 1980. Quantity and quality of Amaranthus grain from plants in temperate, cold and hot, and subtropical climates--A review. In "Proceedings of the Second Amaranth Conference," p. 48. Rodale Press, Emmaus, PA.
Carlsson, R. 1983. Nitrate and oxalate contents in leaf and stem of wild and cultivated leafy vegetables--Leaf nutrient concentrate production as a means of detoxification. Paper presented at 6th World Congress of Food Science and Technol. Dublin, Ireland Sept.
Cheeke, P.R., Carisson, R., and Kohler, G.O. 1981. Nutritive value of leaf protein concentrates prepared from Amaranthus species. Can. J. Animal Sci. 61: 199.
Chidambaram, N. and Ramachandra Iyer, R. 1945. Chemical examination of the seeds of Amaranthus gangeticus. Part 1. The fatty oil from the seeds. J. Indian Chem. Soc. 22:117.
Connor, J.K., Gartner, R.J.W., Rung, B.M., and Amos, R.N. 1980. Amaranthus edulis: An ancient food source reexamined. Austral. J. Exp. Animal Husbandry 20: 156.
Daloz, C. 1980. Amaranth as a leaf vegetable: Horticultural observations in a temperate climate. In "Proceedings of the Second Amaranth Conference," p. 68. Rodale Press, Emmaus, PA.
Del Valle, F.R. 1984. Personal communication. Univ. of Chihuahua, Chihuahua Mexico.
Der Marderosian, A.D., Beutler, J., Pfendner, W., Chambers, J., Yoder, R., Weinsteiger, E., and Senft, J. 1980. Nitrate and oxalate content of vegetable amaranth. In "Proceedings of the Second Amaranth Conference," p. 31. Rodale Press, Emmaus, PA.
Deshpande, P.D. and Rao, M.V.R. 1954. Nitrogen complex and amino-acid composition of amaranth and aconite bean. Ind. J. Med. Res. 42: 77.
Devadas, R.P., Chandrasekhar, V., and Kumari, K.S. 1973. Availability to school children of iron from Amaranthus cooked in two different utensils. Indian J. Nutr. Dietet. 10: 223.
Downton, W.J.S. 1973. Amaranthus edulis. World Crops 25: 20.
Early, D.K. 1977. Cultivation and uses of amaranth in contemporary Mexico. In "Proceedings of the First Amaranth Seminar," p. 39. Rodale Press, Emmaus, PA.
Elias, J. 1977. Food composition table for comparative nutrient composition of amaranth greens and seeds. In "Proceedings of the First Amaranth Conference," p. 17. Rodale Press, Emmaus, PA.
Ellis, B.E. 1973. Catabolic ring-cleavage of tyrosine in plant cell cultures. Planta 111:
Erwin, A.T. 1934. Alegria--A popping seed used in Mexico as substitute for popcorn Iowa State Coll. J. Sci. 9: 661.
Fafunso, M. and Bassir, O. 1976. Nutritional qualities of some African edible leafy vegetables. Effect of boiling on the essential amino acid composition of their extracted protein. J. Food Sci. 41: 214.
FAO. 1973. Energy and protein requirements. Nutrition Meeting Report Series No. 52. Food and Agric. Org., Rome.
Feine, L.B., Harwood, R.R., Kauffman, C.S., and Senft, J.P. 1979. Amaranth: Gentle giant of the past and future. In "New Agricultural Crops," ed. G.A. Ritchie, p 41. Westview Press, Boulder, CO.
Fernando, T. and Bean, G. 1984. Fatty acids and sterols of Amaranthus tricolor L. Food Chem. 15: 233.
Flores, H.E. and Galston, A.W. 1980. Tissue culture of amaranth: Initial results and prospects. In "Proceedings of the Second Amaranth Conference," p. 105. Rodale Press, Emmaus, PA.
Flores, H.E., Thier, A., and Galston, A.W. 1982. In vitro culture of grain and vegetable amaranths (Amaranthus spp.). Am. J. Bot. 69: 1049.
Goering, K.J. 1967. New starches. II. The properties of the starch chunks from Amaranthus retro)qexus. Cereal Chem. 44 245.
Grubben, G.J.H. and van Sloten, D.H. 1981. "Genetic Resources of Amaranths," Intl. Board for Plant Genetic Resources, Food and Agric. Org., Rome.
Haas, P. and Kauffman, C.S. 1984. Grain amaranth: an overview of research and production methods. Regenerative Agric. Assoc., Emmaus, PA.
Haughton, C.S. 1978. "Green Immigrants: Plants that Transformed America." Harcourt Brace Jovanovich, New York.
Hill, R.M. and Rawate, R.I). 1982. Evaluation of food potential, some toxicological aspects and preparation of a protein isolate from the aerial part of amaranth (Pigweed). J. Agric. Food Chem. 30:465.
Irving, D.W., Betschart, A.A., and Saunders, R.M. 1981. Morphological studies on Amaranthus cruentus. J. Food Sci. 46: 1170.
Jain, S.K. and Hauptli, H. 1980. Grain amaranth: A new crop for California. Agronomy Prog. Rept. 107, Agric. Exp. Station, Univ. of California, Davis.
Jathar, V.S., Deshpande, L.V., Kulkarni, P.R., Satoskar, R.S., and Rege, D.V. 1974. Vitamin B12-Like activity in leafy vegetables. Indian J. Biochem. Biophys. 11: 71.
Kauffman, C.S. and Haas, P.W. 1983. Grain amaranth: A crop with low water requirements and high nutritional value. In "Environmentally Sound Agriculture," ed. W. Lo~keretz, p. 299. Praeger Press, New York.
Keshinro, D.O. and Ketiku, A.O. 1979. Effect of traditional cooking methods on the ascorbic acid content of some nigerian leafy and fruit vegetable. Food Chem. 14: 303.
Kramer, A. and Kwee, W.H. 1977. Utilization of tomato processing wastes. J. Food Sci. 42:212.
Lakshminaravana, G. Pantulu, A.J., and Rao, K.S. i984. Lipid class and fatty acid composition of young Amaranthus gangeticus L. Ieaves. J. Agric. Food Chem. 32: 1361.
Lorenz, K. 1981. Amaranthus hypochondriacus--Characteristics of the starch and baking potential of the flour. Starch 33: 149.
Lorenz, K. and Wright, B. 1984. Phytate and tannin content of amaranth. Food Chem. 14: 27.
Martin, F.W. and Ruberte, R.M. 1979. Edible leaves of the tropics. U.S. Dept. of Agriculture, Mayaguez Inst. of Tropical Agriculture, Mayaguez, Puerto Rico.
Martin, F.W. and Telek, L. 1979. Vegetables for the hot humid tropics. Part 6: Amaranth and Celosia. U.S. Dept of Agric., New Orleans, LA.
Marx, J.L. 1977. Amaranth: A comeback for the food of the Aztecs? Science 198: 40.
Mathai, P.J. 1978. Amaranthus: A neglected vegetable. Indian Farming, April, p. 29.
McKell, C.M. 1983. Genetic resources of unexploited native plants. Plant Molec. Biol. Reporter 1: 89.
McMasters, M.M., Baird, P.D., Holzapfel M.M., and Rist, C.E. 1955. Preparation of starch from Amaranthus cruentus seed. Econ. Bot. 9: 300.
NAS. 1975. "Underexploited Tropical Plants with Promising Economic Value," Natl. Acad. of Sciences, Washington, DC.
NAS. 1984. "Amaranth: Modern Prospects for an Ancient Crop." Natl. Acad. of Sciences, Washington, DC.
Obrenovic, I.S. 1983. Effect of purine derivatives on light induced betacyanin formation in Amaranthus. Biochem. Physiol. Pflanzen 178: 625.
Oke, O.L. 1980. Amaranth in Nigeria. In "Proceedings of the Second Amaranth Conference," p. 22. Rodale Press Emmaus, PA.
Oke, O.L. 1983. Amaranth. In "Handbook of Tropical Foods," ed. H.T. Chan Jr., p. 1. Marcel-Dekker, Inc., New York.
Okiei, W. and Adamson, I. 1979. Nitratenitrite, vitamin C and in-vitro methemoglobin formation from some vegetables. Nutr. Rep. Intl. 19: 241.
Okuno, K. and Sakaguchi, S. 1981. Glutinous and non-glutinous starches in perisperm of grain amaranths. Cereal Res. Commun. 9: 305.
Okuno, K. and Sakaguchi, S. 1982. Inheritance of starch characteristics in perisperm of Amaranthus hypochondriacus. J. Heredity 73:467.
Oliveira, J.S. and de Carvalho, M.F. 1975. Nutritional value of some edible leaves used in Mozambique. Econ. Bot. 29: 255.
Opute, F.I. 1979. Seed lipids of the grain amaranths. J. Ex~. Bot. 30:601.
Pal, M. and Khoshoo, T.N. 1974. Grain amaranths. In "Evolutionary Studies in World Crops," ed. J. Hutchinson, p. 129. Cambridge Univ. Press, Cambridge, England.
Piatelli, M., Denicola, M., and Castrogiovanni, V. 1969. Photocontrol of amaranthin synthesis in Amaranthus tricolor. Phytochemistry 8: 731.
Rawate, P.D. 1983. Amaranth (Pigweed): A crop to help solve the world protein shortage. In "Environmentally Sound Agriculture," ed. W. Lockeretz, p. 287. Praeger Press, New York.
Sanchez-Marroquin A. 1980. Potencialidad agroindustrial dei amaranto. Centro de Estudios Economicos Sociales del Tercer Mundo, San Jeromino Lidice, Mexico.
Sanchez-Marroquin, A., Maya, S., and Luis Perez, J. 1980. Agroindustrial potential of amaranth in Mexico. In "Proceedings of the Second Amaranth Conference," p. 95. Rodale Press, Emmaus, PA.
Sauer, J.D. 1950a. Amaranths as dye plants among the Pueblo peoples. Southwest J. Anthropol. 6: 412.
Sauer, J.D. 1950b. The grain amaranths. A survey of their history and classification. Ann. Missouri Bot. Gardens 37: 561.
Sauer, J.D. 1977. The history of grain amaranths and their use and cultivation around the world. In "Proceedings of the First Amaranth Seminar," p. 9. Rodale Press, Emmaus, PA.
Sauer, J.D. 1979. Grain amaranths. In "Evolution of Crop Plants," ed. N.W. Simmonds, p. 4. Longman London.
Saunders, R.M. and Beciker, R. 1984. Amaranthus: A potential food and feed resource. In "Adv. Cereal Sci. Technol," Vol. VI, ed. Y. Pomeranz, p 357. Am. Assn. Cereal Chemists, St. Paul, MN.
Schmidt, D. 1977. Grain amaranth: A look at some potentials. In "Proceedings of the First Amaranth Conference," p. 121. Rodale Press, Emmaus, PA.
Schmidt, P.R. 1971. Comparative yields and composition of eight tropical leafy vegetables grown at two soil fertility levels. Agron. J. 63: 546.
Senft, J.P. 1980. Protein quality of amaranth grain. In "Proceedings of the Second Amaranth Conference," p. 43. Rodale Press Inc., Emmaus, PA.
Stafford, W.L., Mugerwa, J.S., and Bwabye R. 1976. Effects of methods of cooking, application of nitrogen fertilizers and maturity on certain nutrients in the leaves of Amaranthus hybridus subspecies hybridus (Green Head). Plant Foods for Man 2:7.
Stobart A.K., Pinfield, N.J., and Kinsman L.T. i970. Effects of hormones and inhibitors on amaranthin synthesis in seedlings of Amaranthus tricolor. Planta 94: 152.
Stone, L.A. and Lorenz, K. 1984. The starch of Amaranthus--Physicochemical properties and functional characteristics. Starch 36:232.
Sugimoto, Y., Yamada, K., and Sakamoto, S. 1981. Some properties of normal and waxy-type starches of Amaranthus hypochondriacus L. Starch 33: 112.
Sumar, L.S. 1983. The small giant. Amaranth Newsletter, Archivos Latino Americanos de Nutricion 2: 1.
Teutonico, R.A. and Knorr, D. 1984a. Plant tissue culture: Food applications and the potential reduction of nutritional stress factors. Food Technol. 38(2): 120.
Teutonico, R.A. and Knorr, D. 1984b. Food potential of amaranth tissue culture. Plant Cell Reports (submitted).
Teutonico, R.A. and Knorr, D. 1984c. Effect
of issue culture methods and medium variations on oxalate, nitrate, and ascorbic acid concentrations of Amaranthus tricolor. Unpublished paper. Univ. of Delaware Newark.
Teutonico, R.A. and Knorr, D. 1985. Nondestructive method for oxalate determination of cultured Amaranthus tricolor cells. J. Agric. Food Chem. 33: 60.
Tomita, Y., Sugimoto, Y., Sakomoto, S., and Fuwa, H. 1981. Some properties of starches of grain amaranths and several millets. J. Nutr. Sci. Vitaminol. 27: 471.
Tovar, L.R. and Carpenter, K.J. 1980. The effects of alkali-cooking of corn and supplementation with amaranth seed on its deficiencies in Iysine and tryptophan. Paper presented at Am. Chem. Soc. Meeting, San Francisco, Aug.
Uzo, J.O. and Okorie, A.U. 1983. Amaranthus hybridus: A potential grain crop for West Africa. Nutr. Rep. Intl. 27: 519.
Vietmeyer, N. 1978. Poor people's crops. Agenda AID 1(8): 12.
Wills, R.B.H., Wong, A.W.K., Scriven, F.M., and Greenfield, H. 1984. Nutrient composition of Chinese vegetables. J. Agric. Food Chem. ;32: 413.
The authors thank R.M. Saunders for helpful comments and Rodale Press, Inc.,
Copyright © 1985. Institute of Food Technologies.
Info Request | Services | Become EAP Member | Site Map
Give us your comments about the EAP site
Ecological Agriculture Projects, McGill University (Macdonald
Campus)
Ste-Anne-de-Bellevue, QC, H9X 3V9 Canada
Telephone:
(514)-398-7771
Fax:
(514)-398-7621
Email: eapinfo@macdonald.mcgill.ca
To report problems or otherwise comment on the structure of this site, send mail to the Webmaster