

EAP Publications | Virtual Library | Magazine Rack | Search | What's new
Join the Ecological Solutions RoundtableInsects and mites are found almost everywhere | and can survive the most extreme conditions 1 found on earth. Their short life span provides relatively greater opportunity for mutation and evolutionary change. But their life-style has considerable drawbacks. Their small size allows relatively little space for the brain and complex organs, limiting behavioral complexity. Their physiology also prevents them from producing body heat so that they must cease activity in cold weather.
Insects and mites are arthropods, a word derived from the Latin for "jointed limbs." This group also includes centipedes, millipedes, and crustaceans such as crabs, shrimps, and lobsters. All arthropods have an exoskeleton, which is a supporting structure on the outside of the body. The exoskeleton is inherently stronger than the endoskeleton of a vertebrate, permitting placement of the muscles in such a way as to facilitate flight. The rigid exoskeleton limits arthropods' capacity for expansion. This necessitates ecdysis (molting), periods during which the arthropods grow so fast they must escape from their old exoskeleton and form a larger one.
Primitive insects (those that have not evolved into a modified form with fewer parts) have three body parts: head, thorax (3-segmented), and abdomen (10 segmented). They have 3 pairs of legs, 1 pair arising from each segment of the thorax, and 2 pairs of wings, 1 pair arising from each of the last 2 segments of the thorax. In the flies, which include some of the most highly evolved insects, the hind wings are reduced to stubby posts, leaving only 1 pair of true wings.
Mites are not insects but arachnids, as are spiders. Arachnids generally have 4 pairs of legs, not 3; they usually have only 2 body parts: a cephalothorax (head + thorax) and an abdomen (Fig. 2). In the order Acari, which includes the mites and ticks, the body may be oval and compact, with the two body regions apparently combined into one.
Mouthparts of most insects are complex and bear little resemblance to our own, but some elements are common and can be generalized according to their function:
Insects can be divided into four types of feeders based on their mouthparts:
Insects feed on a great variety of living, dead, and decomposing animals and plants and their products; in some cases, blood or plant juices may constitute the entire food supply. Insects' digestive systems are geared to the kinds of foods they eat. Eating habits may differ greatly in a group of insects and also between larvae and adults. Some adults do not feed at all.
Another trait contributing to insect and mite survival is metamorphosis, the process of changing from one form into another. Each form is known as a stage; one stage can be specialized for hibernation, another for feeding and growing, and another for reproduction and dispersal. Insects can exploit different habitats in each stage; for example, a dobso fly spends its immature stages as a predacious underwater insect and its adult phase as a winged nonaquatic form that rarely feeds.
Insect metamorphosis-In simple metamorphosis, the insect passes through three stages: egg, nymph, and adult (Fig. 7). Wings, if present, develop externally during the immature stages. The immature, called nymphs (Fig. 8), generally look like small adults. There is no resting stage before the last molt to the adult stage. Insects exhibiting simple metamorphosis include cockroaches, grasshoppers, crickets, termites, lice, thrips, "true bugs" (such as stinkbugs, plant bugs, water bugs), leafhoppers, aphids, psyllids, and scale insects. The nymphs of some aerial insects (dragonflies, stoneflies, and mayflies), which are aquatic and breathe through gills, are called naiads.
Insects that undergo complete metamorphosis pass through four stages: egg, larva, pupa, (Fig. 9) and adult (Fig. 10). Wings, if present, develop internally during the immature stages; immatures, called larvae, are often completely different in appearance from the adults. Most notably, a resting or pupal stage occurs before the last molt to the adult stage. This pupa can occur inside a cocoon or chrysalis, as in many butterflies; in a smooth pupal case, as in many flies; within accumulations of sticks or soil, as in some beetles; or virtually out in the open. Insects exhibiting complete metamorphosis include beetles, lacewings, butterflies and moths, flies, fleas, ants, wasps, and bees. Larvae of insects exhibiting complete metamorphosis may take different forms (Fig. 11):
Physiology of metamorphosis-An understanding of the process of metamorphosis in insects and mites will help in the development of control strategies. Three different hormones are involved in metamorphosis. It is initiated and controlled by a brain hormone. Ecdysone, also called molting hormone, promotes growth and induces the molting sequence. Juvenile hormone (JH) promotes larval or nymphal development and prevents metamorphosis. JH is produced by glands that are active during the early instars and cease activity in the last pre-adult instar. Its absence in this instar results in metamorphosis.
Several (JH) analogs are being developed as IGR (insect growth regulator) insecticides, including fenoxycarb (Insegar, used in Europe) and methoprene (the mosquito larvicide Altosid). Another insecticide with IGR activity is diflubenzuron (Dimilin), which mimics the action of ecdysone.
Insects normally reproduce only once during their lifetime. Natural populations usually consist of individuals about the same age, with little or no generational overlap. Parthenogenetic reproduction, which does not require a combining of gametes from both sexes, is common in bees, wasps, and roaches. The life cycle of some insects alternates between bisexual and parthenogenetic reproduction (e.g., many aphids). Most insects lay eggs. In some cases, such as in aphids and numerous flies, live young hatch just before or while eggs are being laid. The adults of such insects may differ in appearance and occur on different host plants from one generation to the next.
Generations-In temperate regions, adults of most insects appear for a limited time during a particular season, and some life stage overwinters in a state of dormancy. Most insects in the United States have a single generation per year (e.g., plum curculio in the North, apple maggot). Many large insects in the North require two or more years to complete their life cycle (e.g., larger beetles, cicadas, and some moths). Many insects have more than one generation per year. Occasionally a species will have more generations in the southern part of its range; some moths and butterflies follow this trend. A few insects, usually rather small species (e.g., aphids and mites) that can complete their life cycle in a few weeks, have many generations a year, as long as weather is favorable.
A period of quiescence, or dormancy, occurs in the life cycle of many insects. This period may last from a few days to several months or even years and may occur at any stage in the life cycle. Visible activity ceases, and physiological processes occur at greatly reduced rates. Winter dormancy is usually called hibernation (e.g., Mexican bean beetle will hibernate for several months if subjected to low temperatures); dormancy during high temperature is called aestivation (e.g., plum curculio in late summer). Dormancy is controlled by environmental and genetic factors. Most insects enter dormancy when an environmental factor, such as temperature, becomes unfavorable; they resume activity when conditions are again favorable.
Some species do not breed continuously throughout the year, even under favorable conditions, but enter dormancy before conditions become unfavorable. This period of diapause is normally broken only when the insect is subjected to low temperature and then returned to a temperature favorable for development. For example, second-generation codling moths remain as larvae in cells under apple bark unless subjected to at least three weeks of below-freezing weather; under normal developmental temperatures, they pupate and complete development. Diapause is controlled in part by photoperiod (daylength). The daylength that triggers diapause differs among species. All codling moth larvae that enter the soil for pupation before a certain date will complete their development and emerge as moths and reproduce, but any entering the soil after that date go into diapause and do not complete their development until the following spring.
Communication Insects communicate with other members of the same species by secreting chemical substances known as pheromones (social hormones) to the outside of the body. Pheromones include sex attractants, the queen substance produced by queen honey bees, alarm substances, aggregation substances, territorial markers, and trail substances used by many ants. They are best known in ants, bees, and termites. Pioneering work in the identification of pheromones of economically important insects has been done at the New York State Agricultural Experiment Station at Geneva by Wendell Roelofs and his group since the late 1960s. These advances have led to the manufacture of synthetic sex pheromones, which are used as lures in sticky traps for monitoring insect presence and population magnitude, as developmental indicators, and in controlling pests by permeating the air to interfere with mate finding behavior. This approach is termed the mating disruption strategy.
Mammals are warm-blooded, developing at a constant rate regardless of the environmental temperature because they are able to maintain an internal temperature that allows their biochemical reactions to progress normally. Insects, which are exothermic, remain at the same temperature as their environment (there is no such thing as cold-blooded). They do not generate body heat and therefore depend on favorable external temperature. At a certain temperature, which varies among species, an insect's biochemical reactions cannot proceed and development stops. This temperature is known as the insect's developmental threshold or developmental base. Charting the ambient temperature makes it possible to track insect development, which is directly proportional to the amount of time accumulated above the developmental threshold (up to some maximum not often reached during the season). We divide this time arbitrarily into heat units or degree-days (DD).
There are different ways to determine the quantity of heat units accumulated, which is equivalent to the area under a temperature versus time graph on a given day. The methods are listed below in order of precision in measuring small changes during the day or departures from idealized heating and cooling trends.
Average or Max/Min Method-This method is the simplest and least precise. It assumes that the daily temperature graph is linear and that the area beneath it is triangular.
Sine Wave (Baskerville-Emin) Method-This method is more precise and assumes that the daily temperature cycle takes the form of a sine wave. The area beneath this curve is determined by integration, which requires knowledge of calculus. This method makes the same use of daily maximum and minimum temperatures and developmental thresh old as does the Average Method. Using the Sine Wave Method tends to accumulate more DDs than the Average Method, particularly during the early part of the season.
Continuous Integration Method-This method is the most precise and requires multiple temperature readings hourly or more frequently throughout the day to obtain a temperature versus time graph that is truly representative of a field situation. The area beneath the curve is still calculated using integration. The data collection is most efficient if handled by a computer.
The following methods are attempts to correlate a pest event or activity with another event that can be measured more precisely. Events in an insect's life cycle often occur after the same heat units have accumulated each year, but many years' observations must be collected to measure this precisely. Degree-days can be used to predict events wherever weather data are available.
Temperature-By monitoring temperature and pest activity simultaneously for many years, it is possible to build up a data base of events and the range of accumulated DDs that correspond with them (see Appendix 1).
Phenology: Some events occur reliably at the same time as other, easily observed biological events in the field; for example, mites hatch from late tight cluster to pink; European apple sawflies lay eggs from late bloom to petal fall. These rules of thumb often draw on the evolved relationships between pests and their hosts (see Appendix 2).
Biofix: This is a distinct, easily monitored event in the life history of an organism, used to fine-tune our predictions of its activity; for example, first flight, first egg laid, first mine observed.
Trapping. Insect trapping is used to determine a biofix, to provide an index of population trends, or to indicate population levels.
Sampling. Insect sampling is conducted to learn about a population in a certain location. A population is all the members of a specific group. It can be general or specific, such as all the mites in an orchard, all the adult mites in an orchard, or all the adult European red mites in the orchard. A sample is a portion of the population that you examine and use to make a best guess about the rest of the population. For example, to estimate the number of mites per leaf for all the leaves in an orchard, you could sample 50 representative leaves, examine all the mites on them, and from that sample make a best guess. To take a representative sample, first be sure that the sample is truly representative of the population of interest. For instance, you cannot take all the sample leaves from a single tree or only from border trees if you are interested in all the trees in the block. Second, select samples randomly so that any one leaf has the same chance of being examined as any other, and that you do not just happen to choose leaves that look bronzed, those that look healthy, or those that are easiest to reach.
A sample is a random variable. Simple examples of random events or observations are rolling a pair of dice or flipping a coin. The labels used to describe the result of such an event (e.g., the number showing on the dice) are called random variables. We can describe random variables by setting a likelihood or probability for each possible outcome. For example, the probability of obtaining a 6 with any roll of a single die is 1 in 6, and the probability of obtaining heads with a coin toss is 1 in 2.
The result of a sample of insects or mites is also a random variable. For example, if we use a sample of 50 leaves to estimate the average number of mites per leaf, this estimate is a random variable that will differ somewhat each time we take a sample. If random variables are analyzed statistically, they can help in understanding insect and mite populations in an orchard.
Random variables are important in making pest control decisions. We sample insect populations to estimate their average, or mean, size. Each sample of a given population will vary, but if they are representative they will be close to some average value. Because any estimate of pest population size using a sample is a random variable, there is always some uncertainty about the population's true size. Despite this uncertainty, the estimate of population size is more likely to be close to the true size than far from it. If you are sampling a population that is in reality greater than some treatment threshold, your sample may tell you it is below threshold. All is not lost, however. If the sample is truly representative, this problem is more likely to occur when the true population size is very close to (that is, slightly above or below) the threshold. This means that, although there is a chance your sample will lead you to make a management error (such as no treatment when the population is actually above threshold), there is less likelihood of this happening as the true population size gets farther from the threshold (Fig. 14).
To illustrate, if your threshold is 5.0 mites per leaf and the true (but unknown to you) mite population in the orchard is exactly 5.0 mites per leaf, there is a 50 percent chance that your sample will indicate that the population is below threshold. If the true population is slightly higher, 5.2 mites per leaf, the chance of a wrong conclusion drops to approximately 40 percent. If it is actually 6.0 mites per leaf, it drops to less than 10 percent, and at 7.0 mites per leaf it drops to zero; that is, at some point when the actual population is above, but still relatively close to, the threshold, you will never estimate it to be below threshold. Because thresholds are only general guidelines, the consequences of making a decision error with a population only marginally greater than threshold are not likely to be too serious. Also, the greater the number of samples, the greater the precision of your estimate.
Samples are either fixed or sequential, which refers to their size, for example, the number of leaves you must examine to reach a conclusion. A fixed sample is more basic and generally conservative. This means that, whereas you may take more samples than you actually need to make a decision, you will never take too few. A sequential sample is a more advanced technique used when there is greater familiarity with the insect's biology (specifically, its distribution in particular field situations). The information from each sequential sample is used to determine whether more samples are needed. This is more time efficient because it allows a rapid decision to be made in extreme cases such as when populations are extremely high or low. When taking a sequential sample, keep in mind that nearness to the edge of the Continue Sampling" band has nothing to do with how close the population is to the threshold. It merely shows how close you are to being able to make a decision based on the number of samples you have taken so far.
The presence/absence method is a refinement of the sample and observation process. In special cases of mites and small insects, such as European red mite on apple leaves, the number of mites per leaf is mathematically related to the number of leaves on which one or more mites are present. By taking numerous samples of leaves and recording whether mites are present and the number of mites per leaf, a table of correlations can be constructed and used for a sequential sampling procedure. The grower or scout can use the number of leaves containing mites to make a decision about the need for treatment.
Threshold. The most common estimate entomologists make from samples of insects is whether enough insects are present in the true population to justify taking action. This is called an action threshold and is based on a comparison of the costs of control versus the probable economic loss if no action is taken. Action thresholds are the results of a complex process required to gather enough information about a pest-crop relationship to predict if and when to take control measures. Behind a thresh old are generalizations about the pest's feeding and reproductive behavior, rate of growth, most susceptible stage of growth, population trends, and the type of injury and costs and benefits of preventing it. As such, it can only serve as a guideline for action in most situations; thus thresholds are usually constructed to be conservative, but many rely on information that is rarely given much thought in practice. For example, a grower will probably not try to estimate the market price of apples or the packout expected from a specific block when deciding whether to spray for leafrollers, although the action thresh old may have been set with some attention to these factors. Action thresholds should always be regarded as management aids, not immovable laws, and they do not replace common sense.
Insects with chewing mouthparts inflict great damage on foliage, causing leaves to be skeletonized, riddled with holes, eaten around the edges, or entirely consumed (e.g., larvae of moths, sawflies, and beetles). Other insects suck sap from leaves, stems, or other plant parts, producing a characteristic spotting or browning, curling, or wilting. Feeding on stems or twigs results in dwarfing or wilting. Damage is caused both by removal of the sap and by injury to the plant tissue (e.g., scale insects, aphids, and true bugs).
Scale insects are usually minute, but if they are abundant enough to encrust bark, twigs, or stems they can kill orchard and shade trees. Aphids produce a curling of the leaves and, when feeding on fruit, may cause it to be stunted or misshapen and may change the sugar content, greatly impairing the flavor. Many insects feed as miners in leaves or as borers in stems, roots, or fruits.
Feeding between the upper and lower surfaces of the leaf may cause as much defoliation as does external feeding. There are about 500 leafmining species in the United States (e.g., spotted tentiform and apple blotch leafminers).
Tunneling causes serious damage. Tunneling insects include codling moth, oriental fruit moth, apple maggot, and plum curculio.
Injection of a chemical into plant tissues while the insect feeds causes abnormal growth (e.g., rosy apple aphid) or produces a gall (e.g., woolly apple aphid). Each species of gall insect produces a characteristic gall on a certain part of a particular plant.
Insects at the larval and nymphal stages (e.g., woolly apple aphid) that live in the soil and attack the underground plant parts cause extensive damage.
Shelters in plants are built by leafrollers and leaf folders, which roll or fold the leaves and tie them with silk, feeding in the shelter so formed. Leaf tiers and webworms tie several leaves or even entire branches together, producing large silken webs or tents.
A few insects injure e plants when they lay their eggs, particularly in stems or fruits (e.g., plum curculio, apple maggot, periodical cicada, tree crickets, leafhoppers).
Biology and impact: RAA is the most damaging of the aphids that attack apple. Its saliva, injected while feeding, is translocated to nearby fruit, causing leaf curling and small, deformed apples. RAA can be distinguished from other aphids by its long cornicles and purple-rose color. It overwinters as an egg on twigs, in bud axils, and in bark crevices. The overwintering eggs of RAA, green apple aphid, and the English grain aphid are oblong and pale green at first, then turn shiny black. RAA nymphs are visible beginning at about tight cluster but are most easily observed at the pink bud stage. The first adults appear around bloom. Second-generation adults appear two to three weeks after petal fall. Some of these move to alternate hosts (such as narrow leaf plantain) and the rest remain in the orchard. The third generation develops by mid-July and moves to alternate hosts. In later summer, adult RAA return to trees to lay eggs.
Decision making Because RAA populations are highly variable, it is important to assess their densities before making a treatment. In past field surveys, approximately 50 percent of the orchards sampled required treatment. Sampling can begin at tight cluster but is better done at pink when RAA nymphs are more easily seen. RAA densities are estimated by sampling 10 fruit clusters from the interior canopy area of 10 trees. Treatment is recommended if 1 infested cluster is found. If you have experience sampling RAA, this is probably too conservative and a threshold of 3 to 5 infested clusters is more appropriate.
Control It is not known how important natural enemies (such as larvae of the fungus gnats, Cecidomyiidae) are in regulating RAA populations. Several pesticides can effectively control RAA when applied at pink; even so, you should use a material that will conserve populations of Typhlodromus pyri, an important mite predator.
Biology and impact: STLM was introduced from Europe in the 1880s. Its host plants include apple, wildcherry, hawthorn, quince, plum, and crabapple. STLM overwinters as a pupa in leaf litter on the ground. Adults emerge at the green tip apple bud stage and lay small, flattened eggs that are deposited singly on leaf undersides. Egg laying begins when leaves unfold after half-inch green, and deposition is nearly complete by the end of the pink bud stage. Its five larval stages are divided into sap-feeders (instars 1 to 3) and tissue-feeders (instars 4 to 5). The first summer-generation adults begin emerging in early June (average date is June 13 + 8 days), and larvae are usually present in early July. Second summergeneration larvae are usually present in late August.
STLM damages only foliage, which the larvae eat and mine. This causes reduced photosynthesis and possibly sequestered nutrients. Foliar damage can cause smaller fruit size, premature drop, and poor color. Damage caused by the first summer generation is usually of greatest concern.
Decision making A sequential sampling plan can be used to classify STLM egg density at pink or the density of sapfeeding mines immediately after petal fall (see Appendixes 3 and 4). Treatment is recommended if eggs average 2 or more per leaf on leaves 2, 3, and 4 of a fruit cluster at pink, or if sap-feeding mines average 1 or more per leaf on these leaves at petal fall. Sampling can be completed in approximately 10 minutes. In recent years, only 16 percent of sampled orchards have required insecticide treatments to control first-generation STLM populations (for sampling procedure, refer to the most current edition of Cornell Pest Management Recommendations for Commercial Tree-Fruit Production).
Control Many parasitoids effectively limit STLM populations in some orchards. Most important are the wasps Apanteles ornigis, Sympiesis marylandensis, and Pnigalio maculipes. Insecticide sprays applied in July and August probably do the most harm to these natural enemies.
All effective leafminer pesticides applied at pink or petal fall are toxic to mite predators. If a block has a history of obliquebanded leafroller damage as well as a problematic STLM population, a pesticide capable of controlling both should probably be applied at petal fall.
Biology and impact: OBLR prefers plants in the Rosaceae family but will feed on many unrelated deciduous trees. This leafroller overwinters as a second or third-instar larva on the tree within closely spun cocoons or hibernacula Larvae become active in the spring when buds begin to open. As foliage pushes from the buds, larvae often tie leaves together and conceal themselves in the resulting chamber. Spring-generation moths emerge in early June (average is June 10 _ 5 days) with peak activity in mid-June. First-generation larvae complete their development in late July or early August. Summer-generation moths begin flying in early August. Second-generation larvae feed primarily on foliage but may cause surface injury to fruit if they are very abundant. After feeding briefly, second-generation larvae enter their winter hibernacula.
Spring-generation larvae may eat away large portions of developing fruit. If the fruit survive, they are misshapen with large, deep cavities of healed-over injuries. Fruit damaged by first-brood larvae generally falls off the tree. In processing blocks, this spring generation of OBLR may cause only small fruit losses (2 to 4 percent).
Decision making During bloom or immediately after petal fall, spring generation larval densities are classified as above or below a treatment threshold using a sequential sampling procedure (see Appendix 5). Treatment is recommended if more than 3 percent of fruit spurs contain live OBLR larvae. Sampling can usually be completed in approximately 10 to 15 minutes (for sampling procedure, refer to the most current edition of Pest Management Recommendations for Commercial Tree-Fruit Production). During recent field surveys, only 28 percent of the blocks sampled have required an insecticide treatment to control the overwintering generation of OBLR.
Control Several parasitoids attack OBLR, but their effectiveness in regulating leafroller populations in commercial orchards is unknown. Most growers favor chemical sprays to reduce damage caused by this insect. A contact insecticide is sometimes applied at half-inch green; larval populations cannot be sampled if OBLR is treated this early in the season. Numerous studies have shown that a single spray of an effective material at petal fall controls fruit damage from overwintered larvae as well as applying two sprays against this brood (pre-bloom plus petal fall). Biological pesticides such as Dipel that are based on the bacterium BaciUus thuringiensis are the only controls compatible with IPM because they are not toxic to natural enemies (especially mite predators).
Biology and impact: TPB adults are active in trees and ground cover from the late half-inch green or early tight cluster bud stage (end of April) until two to three weeks after petal fall. Adult populations peak at about the pink bud stage. Eggs are laid primarily in developing fruit starting at bloom, although some oviposition may occur in buds before bloom. The larvae (Fig. 8D) and adults damage fruit by feeding and laying eggs throughout the bloom and early fruit development periods. Little damage is done to mature trees after June, but nursery stock can be damaged throughout the year.
Early damage, which starts on buds at tight cluster and continues through early pink, is relatively unimportant because damaged buds abort before pollination. Later damage during early bloom and near petal fall is more serious because it causes deeper scars in the fruit and the damaged fruit tends to remain on the tree.
Decision making Monitoring for TPB is not recommended in New York because even the most effective insecticide treatments usually cannot completely prevent TPB injury, and damage in treated plots is often similar to that in untreated blocks. Studies by Rick Weires in the Hudson Valley have shown that cullage attributable to TPB injury in the packing house is usually very low (<0.5 percent). Therefore, it is generally not economically profitable to treat for TPB in New York orchards. In New England, adults are monitored with white sticky board traps, and sprays are recommended if an average of 2.4 adults per trap are captured through the tight cluster stage, or if an average of 4.1 adults per trap are captured through pink. For New York populations of TPB, however, the numbers of adults or bleeding and damaged buds cannot be related to the level of potential fruit injury observed at harvest.
Control Natural enemies of TPB include true bugs (nabids, geocorids), ladybird beetles, spiders, and parasitic wasps, but they cannot effectively control this pest in commercial orchards. TPB is difficult to control with insecticides because of its habits and life cycle. Adults are active throughout bloom, so a long period of residual insecticide protection is needed, especially during a prolonged bloom period. Adults are mobile and continually re-infest trees from the ground cover and weed hosts outside the orchard. Synthetic pyrethroids have been the most effective chemicals in controlling TPB. Chemicals applied too early (at half-inch green or early tight cluster) are not effective, probably because residues do not last throughout bloom. Initial tests in New York showed that prepink treatments were best in preventing TPB damage to fruit. Later trials have shown that the relative effectiveness of treatments applied at late tight cluster, prepink, and full pink may vary from year to year.
Biology and impact: ERM overwinters as an egg on the tree. Egg hatch is usually closely correlated with tree phenology, ordinarily beginning at early pink and continuing into bloom. If egg hatch does not coincide with pink, it is usually delayed and starts during early bloom. ERM adults normally appear by petal fall, but few eggs are laid by the first generation of adults on leaves until the first week after petal fall.
Early-hatching ERM nymphs feed on older fruit cluster leaves and may cause bronzing by petal fall if populations are high. Early-season damage before petal fall is usually insignificant, but some studies have shown that heavy damage in early to mid-June can reduce yields during the next season.
Decision making Natural mortality of overwintering eggs is usually substantial but highly variable (10 to 60 percent). Therefore, no sampling or rating scheme has been developed in New York using the density of overwintering ERM eggs to predict the potential early season severity of ERM in commercial orchards. During late bloom and petal fall, ERM is concentrated on older fruit cluster leaves, and therefore the overall density of the first generation will be overestimated by counting mites on the oldest leaves at that time. Early control of ERM is essential in our current IPM programs to prevent early-season damage during June. One method used to quantify mite presence is the mite-day concept, which measures the number of mites and the period of time they are present on the leaves. One mite-day is equivalent to an average of 1 mite feeding on a leaf for 1 day; thus 10 mite-days can be accrued by I mite feeding on a leaf for 10 days or 10 mites feeding for 1 day. Our current economic threshold of approximately 550 total mite-days (for the growing season) assumes that no significant accumulations of mite-days occur before mid- to late June. Therefore, a protective prebloom oil treatment is currently recommended for control of early-season ERM.
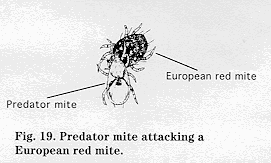
Control Adults of Typhlodromus pyri, a major predacious mite species, are present in the tree at about the time of ERM hatch. These predators control low to moderate densities of ERM but do not regulate high populations. If T. pyri populations in commercial orchards are not destroyed by pesticides, they will control ERM and eliminate the need for acaricide treatments. Amblyseius fallacis, the major predacious mite species in the Hudson Valley, overwinters both in apple trees and in the ground cover beneath them; ground cover, however, appears to have little influence on number and movement of A. fallacis in the tree. A. fallacis was previously believed to be a poor biological control agent because it did not move into trees until late in the growing season after ERM had reached problem levels. More likely, A. fallacis numbers often remain low until late in the season because pesticides toxic to them are used early in the season.
For chemical control, we recommend petroleum oil (2 percent at half-inch green or 1 percent at tight cluster) as an early-season IPM program. Oil is relatively safe to predators, relatively economical, and ERM populations have never shown resistance to it. Furthermore, a thorough coverage of oil applied before foliage is fully developed can kill nearly all the eggs present.
Other early-season treatment options include contact miticides applied at pink or petal fall. Some miticides may be destructive to predacious mite populations and cover less of the foliage present by this time, so this is a less desirable alternative.
Biology and impact: WALH overwinters as an egg beneath the bark surface of one- to five-year-old wood. Overwintered eggs hatch from late pink to petal fall. Nymphs feed on older cluster leaves from pink to petal fall. Both nymphs and adults (Fig. 7) remove chlorophyll from the leaves, causing a white mottling.
Decision making Normally it is not necessary to sample for the first generation of WALH in western New York or the Champlain Valley because damage is not severe and populations are usually relatively low. If petal fall sampling is necessary in certain locations such as the Hudson Valley, the suggested economic threshold is an average of 1 nymph per leaf on 10 fruit cluster leaves from each of 5 to 10 trees.
Control Natural enemies of WALH include several parasites, predators, and a fungus. Unfortunately, they are normally killed by pesticides applied in commercial orchards, so chemical treatments are necessary to control this pest. Because eggs hatch after pink, applications of insecticides, even synthetic pyrethroids, at pink do not have sufficient residual activity to control the first generation of WALH. Several materials can effectively control WALH at petal fall, but some are toxic to predacious mites.
Biology and impact PC adults move into orchards from overwintering sites in hedgerows or the edges of woods and are present in the trees from late pink to early bloom before the fruit is susceptible to damage. Adults are active in the spring when temperatures exceed 60°F. Adult females oviposit in fruit during both day and night but feed mostly at night. Depending on temperature, overwintering adults remain active for two to six weeks after petal fall. Although adults may feed on blossoms, apples are not susceptible to damage until petal fall, at which time adults damage fruit by both feeding and ovipositing. Unlike fruit injured by other pests, many apples damaged by PC will remain on the tree until harvest. Because adults are not highly mobile, orchards near overwintering sites, woodlands, and hedgerows are most susceptible to attack. Fruit damage is usually most common in border rows next to sites where adults overwinter.
Decision making Monitoring for PC is not currently recommended in New York because of the amount of time and labor involved and because PC is generally assumed to be present in every orchard. Various techniques have been used in other areas to monitor PC damage and the presence of adults:
Control Several species of wasps parasitize eggs and larvae of PC. Ants, lacewings, and ground beetles prey on larvae in the soil, and some fungi kill larvae. These organisms are not usually sufficient to regulate populations of PC in commercial orchards.
PC is difficult to control completely with insecticides. Relatively high rates and persistent applications are important because adults may be active for two to six weeks after petal fall depending on temperatures. Several commercial products are available to control this insect. In normal orchards that are not near woodlots or hedgerows and have not suffered previous damage, a single application at petal fall will provide seasonal control. In problem orchards, a petal fall application followed by a second spray 10 to 14 days later will provide adequate control. In orchards with chronic problems, or in seasons when adult activity is prolonged by unusually cool and wet weather, two cover sprays applied 10 to 14 days apart after petal fall may be necessary to prevent late damage.
Biology and impact: EAS overwinters in a puparium in the soil, and adults emerge at the beginning of bloom. Eggs are laid in blossoms at the base of the stamens and hatch in one to two weeks.
Larvae feed below the skin near the apple calyx in a spiral pattern that will cause scarring around the circumference of the fruit at harvest. The larvae then molt and feed deeper inside the apple, causing the fruit to abort. Sawfly damage can be distinguished from that of internally feeding lepidopterous larvae because sawfly exit holes are covered with reddish-brown frass pushed out by the feeding larva.
Decision making This insect is generally a pest only in eastern New York. Because adults are visually attracted to apple blossoms, sticky-coated white rectangles that are non-UV-reflecting can be used to monitor adults. In Massachusetts, a spray is recommended at petal fall if more than an average of 6 to 9 EAS per trap are captured by petal fall in an orchard that received prebloom insecticide, or 4 to 5 in an orchard that did not receive prebloom insecticide. We do not recommend monitoring for this pest in New York because it is normally controlled by the initial spray applied at petal fall to control the plum curculio.
Control: EAS is an introduced pest and its natural enemies in the United States have been little studied. In commercial orchards, EAS is usually controlled by a single spray applied at petal fall. Most insecticides that are effective against the plum curculio, particularly the organophosphates, also control EAS.
Biology and impact CM overwinters as a larva in a cocoon under loose bark on the tree trunk. Adults emerge during bloom, and the first flight continues until about 30 days past petal fall. Eggs, laid singly on the upper surface of leaves or fruit, start to hatch at petal fall and continue for two to three weeks. Larvae feed only on fruit. Surface bites, referred to as stings, cause blemishes; deeper injuries are caused by feeding inside the fruit. Fruits injured by extensive internal feeding usually drop in the middle of June at which time early-season damage becomes noticeable.
Decision making Adult males can be captured in pheromone traps, but numbers of males captured in these traps cannot be related to potential fruit damage. Thus pheromone traps are used only to monitor the seasonal activity patterns of adults within an area. Developmental models, based on temperature accumulations after the first catch of males, can be used to predict the first egg hatch of CM; this approach is used to time initial control sprays for CM in the western United States.
Control The codling moth is attacked by both parasites and predators, but these natural enemies cannot effectively control this pest in commercial orchards. To kill the larvae before they enter the fruit, chemical sprays for CM must be initiated before eggs hatch. In New York, the first generation of CM is normally controlled by sprays for PC at petal fall so special sprays are not necessary. CM is most effectively controlled by the same conventional insecticides used against the plum curculio (organophosphates and synthetic pyrethroids). CM can also be controlled by biorational pesticides such as bacteria (Bacillus thuringiensis), insect growth regulators, viruses, and botanicals, although many of these products are less effective than standard insecticides.
Biology and impact Of these two spider mite species that are usually found in commercial apple trees, ERM is usually the more problematic. Management is similar though their biologies are quite different. ERM feeds primarily on plants in the Rosaceae family (apple, pear, peach), whereas TSSM feeds on a wide variety of host plants (apple, corn, clover). ERM overwinters on apple trees in the egg stage. Eggs hatch between tight cluster and pink. TSSM overwinters as an adult beneath the bark and on other plants in and outside of the orchard. Under favorable conditions, TSSM increases more rapidly than does ERM, but the latter is generally more common. The first adults can usually be found along with the first summer eggs at petal fall. Succeeding generations develop in 10 to 14 days. Overwintering eggs may be laid over a wide time interval, depending on environmental conditions, and are usually laid earlier in populations with high densities.
ERM damages apple leaves by inserting its mouthparts to feed on plant juices. This injury reduces the capacity of the leaf to use sunlight as an energy source (photosynthesis), which may lead to reduced yield and fruit quality. Recent studies of ERM impact in western New York found that the only effect of moderate ERM injury during the mid to late season was a reduction in the color of Red Delicious apples. These results have identified the densities of mites that can be tolerated at various times of the growing season.
Decision making The need for a miticide to control ERM can be determined from a sample of the mite population in an orchard. A sampling procedure is available that determines mite presence based on examination of leaves of intermediate age (see Appendixes 6 8). This procedure divides mite populations into three categories: greater than threshold, below threshold, and much below threshold. The last two categories provide an indication of when the population must be sampled again. If the density is much below threshold, the population should be sampled in 11 to 16 days. If it is simply below threshold, it should be sampled again in 6 to 10 days. If mite predators are present, these intervals can be lengthened by approximately 50 percent.
Sampling involves recording on a chart the presence or absence of mites in distinct samples of leaves and continues until a decision on whether to treat is reached. From petal fall until June 30, a threshold of 2.5 mites per leaf is used; from July 1 to 31, the threshold is 5 mites per leaf; and from August 1 to 15, a threshold of 7.5 mites per leaf is used. Treatment for mites is not currently recommended after mid-August. Adherence to these thresholds will prevent serious injury.
Control: ERM is an induced pest in commercial apple orchards. This means that pesticides used against other arthropods usually destroy naturally occurring mite predators, allowing ERM numbers to increase to damaging levels. Five major predators of ERM are found in commercial New York orchards:
Relatively few miticides can control ERM during the summer. The available contact miticides must be chosen by their individual performance traits, including their activity against specific mite stages and beneficial arthropods, rate of action and length of residual effectiveness, optimal application conditions for each, and any possible resistance that may be exhibited by local mite populations. Because of the limitations of all contact miticides, good spray coverage is essential.
Some research has been done on the use of highly refined petroleum oils to control summer mite populations. Acceptable season-long control has been achieved by using a three-spray program starting at petal fall, followed by periodic monitoring throughout the summer. Potential difficulties with this approach include leaf damage and incompatibility with some of the fungicides used to control summer diseases.
Impact The injury caused by the second and third generations is identical to that caused by the first, but second generation injury is most damaging to the tree. Third-generation STLM is usually not a problem if the second generation was controlled properly.
Decision making Proper timing is essential for both the assessment of STLM densities and control, if required. If done too early, sampling will underestimate the population. If control is applied too late, it will not be effective (see Appendix 9).
Sampling for sap-feeding mines should be done at approximately 690 degree-days (base 43°F) after the start of the flight of the second generation. On average, second-generation STLM moths begin flying about June 13 (+8 days). If moth trap data or DD readings are not available, July 9 is a rough approximation of the appropriate sampling time. Although this procedure may require as many as three separate sampling sessions to determine properly the need for a treatment, the total time spent sampling a given block should not exceed 30 minutes.
A decision regarding the third generation is generally not required unless the density of the second brood exceeded two mines per leaf. In recent years, approximately 8 percent of sampled orchards have required a treatment for second-generation STLM.
Control Several insecticides are effective against second-generation STLM. Unfortunately, all available products are highly detrimental to predatory mites. Depending on the product chosen, application can be made anytime from initial egg deposition until larvae enter the tissue-feeding stages. Sampling is, of course, recommended before any spray is applied.
Impact The principal impact of summer-generation OBLR is its feeding damage to the fruit. This generally occurs if a leaf is webbed to an apple or clustered apples touch each other. Feeding areas on the fruit are shallow, irregular, and may range from small punctures to large excavations. This injury is more serious than that caused by the overwintering generation because most injured fruits remain on the tree.
Decision making Proper timing is essential for both assessment of OBLR densities and control, if required. If done too early, sampling will underestimate the population. If control is applied too late, it will not be effective. Timing is even more critical with OBLR than with STLM (see Appendixes 5 and 10).
Sampling should take place approximately 600 DD (base 43°F) after the start of the first summer flight. On average, summer-generation OBLR moths start flying the first or second week of June. The value of knowing the precise date of this event on your own farm cannot be emphasized too strongly. If degree-day estimates are not available, sampling should be conducted approximately 24 days after the first adult moth is caught. If information on adult moth flight is not available, July 5 to 10 is a rough approximation of the appropriate sampling period. At 600 DD after the start of the adult flight, populations are classified into one of two density categories:
Biology and impact Adults from the second or summer generation of CM start to fly about mid-July, and the peak flight in western New York occurs around the first week in August. Larvae from this generation are active in fruit throughout August.
Larvae from the second generation damage fruit as described in the section on early-season pests. Fruit damage by the second generation is generally more serious than that of the first.
Decision making Males can be monitored with pheromone traps to determine flight activity, but it is not possible to correlate trap catch with potential damage. It is not practical to monitor commercial apple orchards for CM eggs or larval fruit entries because of the theoretical zero tolerance for internal fruit damage.
Control The codling moth is attacked by both parasites and predators, but these natural enemies cannot effectively keep this pest at acceptable levels in commercial orchards. In New York, the second generation of CM is normally controlled by the same conventional insecticides used on apple maggot, so no special sprays are required in most commercial orchards.
Biology and impact It was previously believed that only WALH (which exhibited two generations after petal fall and in mid- to late August) and potato leafhopper (which appeared sporadically between these broods, depending on weather) were present in New York apples. An apparent additional brood has been noted in eastern New York between July and early August. This brood tends to overlap the late August population, so that various stages of WALH are often found on leaves throughout the summer. Recent field observations have shown that many of the leafhoppers seen in apples during midsummer may be a closely related species, rose leafhopper. An initial study of the leafhopper species complex in the Hudson Valley showed that RLH completes its first generation on weed hosts such as multiflora rose; adults begin ovipositing on apple in mid-June, and nymphs appear by early July. From this time until harvest, both species are likely to be present on apple trees; usually one greatly predominates over the other, but the factors influencing the species mixture have yet to be determined. WALH (or leafhopper species complex) appears to have two fairly distinct generations in western New York. Eggs from the single summer generation usually begin to hatch from late July to early August, continuing until mid- to late August. Adults appear in late August and are active until fruit harvest.
Nymphs and adults feed on leaves during the summer, removing chlorophyll and causing white stippling. Excrement from nymphs and adults on fruit leaves small black spots that resemble the summer disease, flyspeck. During harvest, adults fly throughout the tree canopy, annoying pickers.
Decision making: WALH nymphs and adults are usually most common on older fruit cluster leaves inside the tree. The number of WALH on a single older fruit cluster leaf should be counted on each of 10 clusters from 5 to 10 trees. Economic threshold levels for WALH feeding damage on apples have not been developed in New York, but the thresholds suggested in other states vary from an average of 0.25 to 2 WALH nymphs and adults per leaf. Treatment for second- or third-generation WALH (or RLH mixture) is recommended in New York if an average of one or more nymphs and adults per leaf is detected.
Control Several parasites, predators, and a fungus attack WALH, but because these natural enemies are normally destroyed by pesticides they cannot adequately control WALH in commercial orchards. Chemical control is usually most effective if treatments are applied primarily against nymphs after most eggs have hatched.
Biology and impact: PLH is generally a more serious problem in the Hudson Valley than in western New York or the Champlain Valley. PLH does not overwinter in the Northeast but instead migrates on thermals (warm air masses) from the South. Adults usually reach the Hudson Valley by May or early June and are found from mid- to late June in western New York. Because PLH migrate constantly during the season, there are no distinct broods or generations and the pest may be present continuously in orchards from June through harvest.
PLH feeds on tender young terminal leaves. Initially, injured leaves turn yellow around the edges, then become chlorotic and deformed (cupping upward) and later turn brown or scorched. Damage is caused by a toxin injected by PLH while feeding. PLH also occasionally causes symptoms similar to the effects of growth regulators, such as excessive branching preceding or beyond the point of extensive feeding. PLH damage is often mistaken for in, ury caused by herbicides, nutrient deficiency, or overfertilization. PLH injury may not be serious on mature trees but can severely stunt the growth of young trees.
Decision making Nymphs and adults should be counted on 50 to 100 randomly selected terminal leaves in an orchard. Older trees should be sampled approximately every three weeks during the summer. Young trees should be sampled weekly from early June through July. PLH nymphs are often characterized as moving sideways like crabs, whereas WALH generally move forward and back. No formal studies have been conducted in New York to determine the economic injury level for PLH on apples, so we suggest a tentative threshold of an average of one nymph or adult PLH per leaf.
Control Little is known about the natural enemies of PLH, but it is assumed that they cannot control this pest in commercial New York orchards. Populations of PLH in New York are resistant to the conventional organophosphate materials. Moreover, many of the pesticides in other chemical classes that are effective against PLH are toxic to beneficial mites.
Biology and impact Although small numbers of these aphids may be present on trees early in the season, populations generally start to increase in mid- to late June. Large numbers of both may build up on growing terminals on apple trees during summer. Both species are apparently common during the summer in New York orchards, although no extensive surveys have been done to compare their relative abundance in different production areas throughout the season.
Nymphs and adults of both species suck sap from growing terminals and water sprouts. High populations cause leaves to curl and may stunt shoot growth on young trees. Aphids excrete large amounts of honeydew, which collects on fruit and foliage. Sooty mold fungi that develop on honeydew cause the fruit to turn black, reducing its quality.
Decision making Aphids should be sampled several times throughout the season starting in mid-June. Inspect 10 rapidly growing terminals from each of 5 trees throughout the orchard. Record the percentage of infested terminals. No formal studies have been done to develop an economic threshold for aphids in New York orchards. Currently, treatment is recommended if 30 percent of the terminals are infested with either species of aphid.
Control The larvae of syrphid and cecidomyiid flies prey on aphids throughout the summer. These predators complete about three generations during the summer. Most insecticides are somewhat toxic to these two predators, and they usually cannot build up sufficient numbers to control aphids adequately in regularly sprayed orchards. Both aphids are resistant to most organophosphates, but materials in other chemical classes control these pests effectively.
Biology and impact: WAA colonizes both aboveground parts of the apple tree and the roots and commonly overwinters on the roots. In the spring, nymphs crawl up on apple trees from the roots to initiate aerial colonies. Most nymphs are born alive to unmated females on apple trees during the summer. Colonies initially build up on the inside of the canopy on sites such as wounds or pruning scars and later become numerous in the outer portion of the tree canopy, usually during late July to early August.
Aerial colonies occur most frequently on succulent tissue such as the current season's growth, water sprouts, unhealed pruning wounds, or cankers. Heavy infestations cause honeydew and sooty mold on the fruit and galls on the plant parts. Severe root infestations can stunt or kill young trees but usually do not damage mature trees. Large numbers of colonies on trees may leave sooty mold on the fruit, which annoys pickers because red sticky residues from crushed WAA colonies may accumulate on their hands and clothing.
Decision making During late May to June, water sprouts, pruning wounds, and scars on the inside of the tree canopy should be examined for WAA nymphs. During mid-July, new growth around the outside of the canopy should be examined for WAA colonies. No economic threshold has been determined for treatment of WAA.
Control: Aphelinus mali, a tiny wasp, frequently parasitizes WAA but is very susceptible to insecticides and thus does not provide adequate control in regularly sprayed commercial orchards. Different rootstocks vary in their susceptibility to WAA The following resistant rootstocks are the only means of controlling underground infestations of WAA on apple roots: MM. 106, MM. 111, and Robusta
WAA is difficult to control with insecticides because of its waxy outer covering and tendency to form dense colonies that are impenetrable to sprays. WM is resistant to the commonly used organophosphates, but other insecticides are effective against WAA
Biology and impact These two species of leafrollers, which have occasionally damaged fruit in the Hudson Valley, have apparently become serious problems in some orchards during the last several years because they have developed resistance to organophosphate insecticides. The variegated leafroller is found from Kingston (in Ulster County) south to the Rockland County line, in a narrow band bordered by the Hudson River on the east and the Marlboro mountain range on the west. The Sparganothis fruitworm is found predominantly in Columbia County on the east side of the Hudson River and north to Albany. It is also prevalent in western New York, but is currently not a pest in commercial apple orchards there.
Both species overwinter as third instar larvae in the orchard ground cover and begin feeding in early spring on weeds and plants under trees. Larvae pupate in the ground cover, and adult moths emerge shortly after petal fall. Adults lay eggs on apple leaves during June; eggs hatch and larvae are found from late June to July. A second flight begins in late July. These larvae may feed on fruit in late summer until they reach the third instar, at which time they spin down into the ground cover to overwinter.
Larvae of the summer generation may use dead leaves to build a feeding shelter beneath the apple. Most of the larvae from the overwintering generation probably feed primarily on leaves in the late summer, but they may occasionally damage fruit. This late-season damage is less extensive than that from the summer generation of larvae but usually consists of tiny pinholes on the fruit surface.
Decision making Males of both species can be monitored in pheromone traps, but numbers caught in the traps cannot be related to potential fruit damage in the orchard. Because these species are serious problems only in certain orchards, the most reliable way to determine if a specific block requires treatment would be to monitor larval populations during June and July. No formal techniques have been developed to sample these larvae. Likewise, no formal studies have been done to estimate an economic threshold level for initiating summer treatments. Usually it would not be considered economically feasible to apply special treatments to control these leafrollers unless at least 3 to 5 percent fruit damage was anticipated. This threshold represents a larger value than the cost of the spray, but leafroller sprays can never completely eliminate damage. Special leafroller sprays may also harm mites and beneficials and could increase the cost of mite management.
Control Several parasites attack leafroller larvae, keeping them to relatively low levels in unsprayed orchards. Because these parasites are susceptible to insecticides, they are not effective in controlling leafrollers in sprayed commercial orchards. Leafrollers in the Hudson Valley are resistant to the commonly used organophosphate insecticides. Other chemicals available for use are the same as those commonly used to control OBLR. Larger larvae are more difficult to kill with these materials, so sprays should be targeted against small larvae whenever possible.

Biology and impact The apple maggot overwinters as a pupa in the soil. Adults from the single generation of flies emerge in late June to early July. Females cannot lay eggs until they become reproductively mature, 7 to 10 days after emergence. Females lay eggs in fruit and larvae develop there, emerging in the autumn after the fruit has fallen and entering the soil to pupate. Flies are active from July to mid-September, but commercial orchards require protection only from about mid-July to mid-August. Flies do not reach orchards in large numbers until mid-July, and before this date fruit remaining on the tree is unfavorable for larval development, so early infestations do not cause sustainable populations in orchards. In addition, for unknown reasons, fly activity between about August 20 and September 15 does not usually cause serious damage in commercial orchards in New York.
Larval tunneling inside fruit causes it to become rotten and unmarketable. Early stings caused by punctures from the female's ovipositor may severely deform the fruit of some varieties, even though no larvae survive.
Decision making Monitoring to determine whether control sprays are necessary is recommended only in orchards that are not near large sources of outside infestation, such as abandoned orchards or those with no indigenous infestations of flies (see Appendix 11). On about July 15, hang three sticky red sphere traps baited with apple volatile lures in trees along the edge of the block closest to an abandoned orchard or a stand of woods. Place traps 30 feet apart and 4 to 6 feet high in the tree. Position them so they are surrounded but not obstructed or touched by fruit and foliage. Check traps one to two times per week. If a cumulative average of 5 AM flies per trap is captured, apply a spray of a suitable insecticide immediately. After spraying, ignore catches for 10 to 14 days because residues will continue to kill any immigrating flies. After this period, begin checking traps and accumulating catches again. Repeat this procedure until August 15 to 20.
Theoretically, there is absolutely no tolerance for AM damage in fruit. In practice, AM damage is not usually detected in normal fruit inspections unless there is approximately 5 percent fruit damage.
Control Small wasps parasitize AM larvae in fruit, and predators such as birds and crickets may eat larvae or pupae in or near the soil. In natural, unsprayed apple and hawthorn trees, AM populations are not regulated by natural enemies. Parasites and predators are also ineffective at controlling AM in commercial orchards.
AM flies have a limited migratory capability, so all apple and hawthorn trees within 1/4 to l/2 mile of commercial orchards should be removed if possible. Do not allow dropped fruit to remain beneath the tree for more than one to two days. Eliminating fruit drops will break the life cycle of flies in an orchard by preventing larvae from exiting the fruit and entering the soil.
AM flies can be trapped in small, well-pruned trees that are not near large sources of outside infestations. A relatively high density of sticky red spheres (plain or volatile-baited) is required, approximately 1 trap per 100 apples. Mass trapping is usually less effective than chemical control, and AM may still damage 1 to 5 percent of fruit from mass-trapped orchards.
Most commercial orchards have no indigenous populations of flies. Therefore, chemical control sprays are usually directed against flies immigrating into orchards from outside, unsprayed hosts, including both apples and hawthorns. Most insecticides, particularly organophosphates, are remarkably effective in controlling adults. Insecticides must kill females before they oviposit in the fruit. Residual effectiveness of insecticides is particularly important in controlling AM in commercial orchards when flies are continuously immigrating.
Insecticides can be applied according to trap catches as described above or on a standard or modified IPM schedule. The standard schedule requires an initial spray 7 to 10 days after the first emergence of flies, followed by additional sprays at 10- to 14-day intervals until August 15 to 20. The modified IPM schedule requires only three sprays on approximately July 15, August 1, and August 15.
During the past twenty-five years, three new diseases of honey bees have been accidentally introduced to North America, two from Europe and one from Asia Data from the New York State Department of Agriculture and Markets show that the number of human-managed colonies in the state has decreased from 120,000 to 70,000 in the past four years. These figures do not include the heavy losses from the winter of 1992-1993. Many individual beekeepers have reported losses of 20 to 80 percent in a single year since 1988.
In many areas of the state, especially where migratory beekeeping is practiced or colonies are moved for pollination and the new diseases are thus more widespread, all or nearly all of the feral colonies living in hollow trees, buildings, or field crates have died because of one or a combination of these diseases. At the same time, the diseases that have been present for many years continue to take a heavy toll.
The first of these new diseases is chalkbrood, which is caused by a fungus that attacks and kills honey bee larvae. Since it was first found in 1968, it has cost beekeepers as much as 5 percent of their honey crop. During the past four years the second of these diseases, caused by mites that infest the breathing tubes of adult bees, has been even more troublesome. However, Asian varroa mites that live externally on pupal and adult bees are rapidly becoming the most serious of the pests, causing many colony deaths in 1992.
Commercial beekeepers, especially those who migrate, have compensated for these disease losses by dividing colonies in Florida in February and March or by buying package bees and queens from a southern state. However, an estimated 40 percent of the hobby beekeepers, who own at least half of the bees in New York State, no longer have live bees. The colonies owned by these hobby beekeepers, as well as the feral colonies, have been important in the pollination of commercial crops, especially apples, but also fruits and vegetables grown both commercially and in home gardens. In some areas solitary ground- and twig-nesting bees and bumble bees will be helpful in pollination, but on a year-to-year basis their populations fluctuate widely and cannot be depended upon.
Good chemical controls for varroa mites are available and are being widely used. Beekeepers report, however, that the chemicals used for tracheal mite control are only marginally effective. It is agreed that the bees being kept today are more tolerant of tracheal mites and chalkbrood, merely because the most susceptible stock has died. Research is under way to develop bees resistant to these new diseases, but we have not reached the stage where specific strains of bees can be recommended.
Managing colonies for maximum flight. The existence of the new diseases increases the importance of care and timing in placing and managing colonies for pollination in orchards. The following guidelines are especially critical.
Honey bees will visit plants with the greatest quantities of pollen and the highest sugar concentrations in the nectar. The nectar of dandelions and yellow rocket is as rich as that of apple. Orchardists should mow flowering weeds in orchards or apply weed killer. Weeds in fields adjacent to orchards may also attract bees away from the trees to be pollinated.
Colonies of honey bees in orchards should be kept in full sunlight to warm the hives rapidly in the morning and entice the workers out of the hives. We suggest placing colonies in groups of three to five to take advantage of the best locations. Good locations should slope to the east or south with entrances facing in these directions and should be protected from the wind. Colonies should be placed on pallets, cinder blocks, old tires, or any objects that will keep the bottom boards six to eight inches above the ground. Hives with wet bottomboards will be cooler, which slows bees' flight. A hive stand will also keep colonies above grass, which may shade or block the entrance.
Bees often collect large quantities of water to dilute the honey they feed their young. It is impractical to carry sufficient water into an orchard or to fill all wheel ruts and holes with dirt or sand and force the bees to forage outside of the orchard for water. But growers must understand that water contaminated with pesticides can kill bees that collect it. A problem exists if more than 10 dead bees are found in front of a hive in the morning. If too many bees die, it may be necessary to rent more bees. Beekeepers expect some losses and figure them into their rental fee.
Pesticides are less of a problem to bees and beekeepers today than they were 10 and 20 years ago. Nevertheless, it is still important to read the label and to avoid using materials that are especially toxic to bees. Honey bees are most often killed by pesticides when they ingest contaminated pollen. Avoid spraying when flowers, including weeds, are open and attractive to bees.
Red Delicious and a few other apple varieties have flower structures that are different from most other common varieties such as McIntosh. Their anthers are widespread, and bees learn to insert their mouthparts between the anthers to obtain nectar. In this way, the bees do not contact the flower's sexual parts and no pollination occurs. It takes time for bees to learn to obtain nectar in this way. To counteract this problem, the number of colonies in the orchard must be increased so there are more bees that have not learned this technique.
New York growers currently use about one colony of bees per three acres for apple pollination. This number may be adequate in small orchards, which may be visited by feral honey bees and solitary and subsocial bees such as bumble bees from adjacent hedgerows and woods. Growers with larger blocks may wish to increase the number of colonies to one per two acres, especially considering the new diseases.
Pollination of pears will probably always be a problem because pear nectar contains only about 15 percent sugar versus 40 percent for apples, dandelions, and yellow rocket. The answer is to move the bees into the center of the pear block when the pears are in full flower. It will take several hours for the bees to discover the better sources farther away, and in that time the pears may be adequately pollinated. An alternative is to use more colonies per acre, which will increase the number of naive bees.
Bees will visit flowers and pollinate only if they can fly. Cool, rainy, and windy weather will delay, slow, or stop flight. In warm years bees may overpollinate during bloom, and growers must thin the flowers. Unfortunately, we cannot predict the weather. For the above reasons, you should contract for bees for pollination well ahead of when the colonies will be needed.
Abbreviations: EMR= Europoean Red Mite; OBLR= Obliquebanded Leafroller; OMF= Oriental Fruit Moth; RBLR= Redbanded Leafroller; STLM= Spotted Tentiform Leafminer
Notes: Information in the above table is based on field abservations. Values and dates are given ±1 standard deviation; i.e., events should occur within the stated range approximately 7 years out of 10. This information is provided as a scouting and sampling guide.
During the pink bud or early bloom stage, start near one corner of the block and go to every other tree until you have sampled enough trees to reach a decision. Select 3 fruit clusters from around the canopy of each tree sampled.
Using a magnifier, count the eggs on the undersides of the second, third, and fourth leaves in each cluster, counting leaves in the order they unfolded (see diagram at right).
After 2 trees have been sampled, begin comparing the accumulated total number of eggs found using the decision lines shown in the chart at right for that number of trees.
If the number of eggs falls between the two stairstep lines, sample another tree. If the total is less than the lower line, sampling is stopped and no treatment is recommended. If the total is greater than the upper line, sampling is stopped and a treatment is recommended at either pink or petal fall. If 7 trees are sampled and the total number of eggs equals 126, the population is below threshold.
If STLM eggs were not sampled during the pink or early bloom stage, a decision on first-generation control can still be made by sampling sap-feeding mines at petal fall. After all the blossoms have fallen, start near one corner of the block and go to every other tree until you have sampled enough trees to reach a decision. Select 3 fruit clusters from around the canopy of each tree sampled.
Using a magnifier, count the mines on the undersides of the second, third, and fourth leaves in each cluster, counting leaves in the order they unfolded (see diagram at right).
After 2 trees have been sampled, begin comparing the accumulated total number of mines found using the decision lines shown in the chart at right for that number of trees.
If the number of mines falls between the two stairstep lines, sample another tree. If the total is less than the lower line, sampling is stopped and no treatment is recommended. If the total is greater than the upper line, sampling is stopped and a treatment is recommended at petal fall. If 7 trees are sampled and the total number of mines equals 63, the population is below threshold.
Examine 10 bud clusters (overwintering generation) or expanding terminals (first summer generation) per tree for live OBLR larvae. For the first summer generation, sample at -600 degree-days (43°F base) after the first moth flight in your area; if you do not have access to this information, use July 5 as an estimated best sample date in western New York (5-7 days earlier in eastern New York and on Long Island).
Sample every other tree starting with a random tree and continuing down the row. Remember that you are not counting OBLR larvae but sites infested with live OBLR. If trees are >10 ft. tall, try to include some samples from the upper canopy or from watersprouts.
If the total number of infested terminals (for first-generation summer larvae) falls between the two stairstep lines, sample another tree. If the total is less than the lower line, sampling is stopped and no treatment is recommended. If the total is greater than the upper line, sampling is stopped and treatment with a suitable material is recommended at petal fall. Refer to the current Pest Management Recommendations for Commercial Tree-Fruit Production for a choice of pesticide materials.
Continue sampling until you reach one of the boldface staircase lines in the chart above, or until you have examined a maximum of 100 clusters. If you reach the intersection of the two lines by the 100th sample, withhold treatment.
Use This Table to help keep track of your samples |
|||||
Total number examined |
# Infested |
Total number examined |
# Infested |
||
| 10 | 60 | ||||
| 20 | 70 | ||||
| 30 | 80 | ||||
| 40 | 90 | ||||
| 50 | 100 | ||||
This procedure involves examining middle-aged leaves for motile mites (any stage except eggs). Use this chart, which corresponds to a mite density of 2.5 mites per leaf, from June 1 until June 30. You will not be counting mites but will only determine whether they are present or absent on each leaf sampled.
Starting with a random tree and sampling every other tree, collect 4 leaves in a plastic bag from each of 5 trees, choosing from each quadrant of the canopy. To make sure the leaves are of intermediate age, pick them from the middle of the fruit cluster.
Using a magnifier, examine the top and bottom surface of each leaf for motile mites, and keep track of the number of leaves containing motile mites. When all 20 leaves have been examined, compare this number with the decision lines on one of the above charts. If you are in either of the "Continue" zones, take more leaf samples in batches of 10 (5 per tree, for simplicity), adding the number with mites present to your original value while checking the chart again. Continue until you have passed out of the "Continue" zone to arrive at a decision. If you reach "Stop sampling and treat," the population is above threshold and a miticide application is recommended. If you reach one of the "Resample" zones, the population is below threshold and should remain so for at least the number of days stated. Return at the designated time and conduct another sample. If the "6- to 10- day" resample date falls during the 5.0 mites/leaf threshold period, you can wait for a total of 11-16 days before resampling.
This procedure involves examining middle-aged leaves for motile mites (any stage except eggs). Use this chart, which corresponds to a mite density of 5.0 mites per leaf, from July l until July 31. You will not be counting mites but will only determine whether they are present or absent on each leaf sampled.
Starting with a random tree and sampling every other tree, collect 4 leaves in a plastic bag from each of 5 trees, choosing from each quadrant of the canopy. To make sure the leaves are of intermediate age, pick them from the middle of the fruit cluster.
Using a magnifier, examine the top and bottom surface of each leaf for motile mites, and keep track of the number of leaves containing motile mites. When all 20 leaves have been examined, compare this number with the decision lines on one of the above charts. If you are in either of the "Continue" zones, take more leaf samples in batches of 10 (5 per tree, for simplicity), adding the number with mites present to your original value while checking the chart again. Continue until you have passed out of the "Continue" zone to arrive at a decision. If you reach "Stop sampling and treat," the population is above threshold and a miticide application is recommended. If you reach one of the "Resample" zones, the population is below threshold, and should remain so for at least the number of days stated. Return at the designated time and conduct another sample. If the "6 to 10- day" resample date falls during the 7.5 mites/leaf threshold period, you can wait for a total of 11-16 days before resampling.
This procedure involves examining middle-aged leaves for motile mites (any stage except eggs). Use this chart, which corresponds to a mite density of 7.5 mites per leaf, from August 1 until August 15. You will not be counting mites but will only determine whether they are present or absent on each leaf sampled.
Starting with a random tree and sampling every other tree, collect 4 leaves in a plastic bag from each of 5 trees, choosing from each quadrant of the canopy. To make sure the leaves are of intermediate age, pick them from the middle of the fruit cluster.
Using a magnifier, examine the top and bottom surface of each leaf for motile mites, and keep track of the number of leaves containing motile mites. When all 20 leaves have been examined, compare this number with the decision lines on one of the above charts. If you are in either of the "Continue» zones, take more leaf samples in batches of 10 (5 per tree, for simplicity), adding the number with mites present to your original value while checking the chart again. Continue until you have passed out of the "Continue" zone to arrive at a decision. If you reach "Stop sampling and treat," the population is above threshold and a miticide application is recommended. If you reach one of the "Resample" zones, the population is below threshold, and should remain so for at least the number of days stated. Return at the designated time and conduct another sample. If the resample date falls after August 15, there should be no further need for additional samples or miticide sprays this season.
Because of variability in this pest's development from one site to the next, more than one sampling session may be needed to reach a treatment decision for second-generation STLM. The first sample should be taken at 690 degree-days (base 43°F) after the start of the second moth flight (or approximately 25-30 days). Use July 9 as an approximate sampling date if you do not have access to pheromone trap catch data.
Start near one corner of the block and sample trees along a diagonal moving toward the opposite corner of the block. At each tree, count all the sapfeeding mines on 4 mature terminal leaves randomly selected from around the outside of the canopy. Sampled leaves should be those located near the middle of the terminals. After sampling 3 trees, start comparing the accumulated total number of mines found using the appropriate chart for the sampling session and proceed as follows:
SAMPLING DONE AT 690-840 DD
If the number of mines falls in the "Continue" zone on Chart 1, sample another tree and check again. If the total is above this zone (area 1), sampling is stopped and a treatment is recommended. If the total is below this zone (area 2), stop sampling and sample the block again at approximately 840 DD (about 31 days) after the start of the second flight.
SAMPLING DONE AT 840-1149 DD, IF NECESSARY
If it is necessary to sample the population a second time, refer to Chart 2 after sampling the third tree. If the accumulated total falls in one of the "Continue" zones, sample another tree and check again. If the count falls in area 1, a treatment is recommended and no further sampling is necessary. If the count falls in area 2, stop sampling and sample the block again at approximately 1150 DD (about 42 days) after the start of the second flight. If the count falls in area 3, treatment is not recommended and no further sampling is necessary.
SAMPLING DONE AT 1150 OR MORE DD, IF NECESSARY
If it is necessary to sample a third time, refer again to Chart 1, the same as in the first sampling session. This time, however, if the accumulated total number of mines falls in area 2, treatment is not recommended, and no further sampling is required for this brood of STLM.
Sample at approximately 600 degree-days (43°F base) after the first adult flight in your area; if you do not have access to this information, use July 5 as an estimated best sample date in western New York (5-7 days earlier in eastern New York and on Long Island).
Examine 10 expanding terminals per tree, selecting trees from as wide an area of the block as possible. If trees are >10 ft. tall, an effort should be made to include some samples from the mid- to upper canopy area, or from watersprouts, which are favored infestation sites. Try not to bias your sample by picking terminals that you suspect are infested.
Record the number of terminals that are infested with live OBLR larvae. Continue sampling until you reach one of the staircase lines on the above chart. If you reach the intersection of the two lines by the 100th sample, this is equivalent to a Don't Treat decision. If you reach a Treat decision, an insecticide currently recommended at this time.
If you reach a Don't Treat decision, return in 3-5 days (100 more degree-days) and repeat the sample; a Don't Treat decision the second time indicates that no treatment is recommended against this generation of OBLR.
Use This Table to help keep track of your samples |
|||||
Total number examined |
# Infested |
Total number examined |
# Infested |
||
| 10 | 60 | ||||
| 20 | 70 | ||||
| 30 | 80 | ||||
| 40 | 90 | ||||
| 50 | 100 | ||||
On or before July 15, hang 3 sticky red sphere traps baited with apple volatile lures in the trees along the edge of your block closest to an abandoned orchard or a stand of woods. If no abandoned trees or woodlands are nearby, choose the southern edge of the block. Traps should be spaced at least 30 ft. from each other, on the outside edge of the canopy, at least 6 ft. high. Position the traps so that they are surrounded by fruit and foliage, but are not touched by them or obstructed from view. Traps should be checked 1-2 times per week for apple maggot flies (AM), which can be distinguished from similar species by the pattern of dark bands on their wings (drawing at right). If a total of 5 AM flies per trap are caught (15 in this case), a spray of a suitable insecticide is recommended immediately, after which the traps can be ignored for 10-14 days.
Begin checking the traps again after this period of protection by the spray residue. Traps should be cleaned of non-pest flies periodically and recoated with stickum if necessary. No treatment is recommended until a cumulative total of 5 AM flies per trap are caught. If unbaited sphere traps are used, the threshold should be lowered to 1 AM fly per trap. Traps can be taken down by August 30.
Date Checked |
Total number of AM flies caught since last spray |
Total AM |
Date of last spray |
Material/ Rate |